* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 16.9 Electric Fields and Conductors
Electron mobility wikipedia , lookup
Nuclear physics wikipedia , lookup
Magnetic monopole wikipedia , lookup
History of quantum field theory wikipedia , lookup
History of subatomic physics wikipedia , lookup
Speed of gravity wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
Condensed matter physics wikipedia , lookup
Fundamental interaction wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Maxwell's equations wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Time in physics wikipedia , lookup
Anti-gravity wikipedia , lookup
Electromagnetism wikipedia , lookup
History of electromagnetic theory wikipedia , lookup
Field (physics) wikipedia , lookup
Lorentz force wikipedia , lookup
Chien-Shiung Wu wikipedia , lookup
Chapter 16
http://www.stmary.ws/highschool/physics/home/videos/hyperphy
sics/jenvan3.mov
Electric Charge and Electric Field
Physics is Life
Units of Chapter 16
Static Electricity; Electric Charge and Its Conservation
Electric Charge in the Atom
Insulators and Conductors
Induced Charge; the Electroscope
Coulomb’s Law
Solving Problems Involving Coulomb’s Law
The Electric Field
Field Lines
Electric Fields and Conductors
Physics is Life
2
Introduction
You walk across the rug, reach for the doorknob
and..........ZAP!!! You get a static shock.
Or, you come inside from the cold, pull off
your hat and......BOING!!! Static electricity
makes your hair stand on end. What is going
on here? And why do static problems only
seem to happen in the winter?
Physics is Life
What is Static Electricity?
Static electricity refers to the buildup of electric charge on the
surface of objects. The static charges remains on an object until
they either bleed off to ground or are quickly neutralized by a
discharge. Although charge exchange can happen whenever any
two surfaces come into contact and separate, a static charge will
only remain when at least one of the surfaces has a high resistance
to electrical flow (an electrical insulator). The effects of static
electricity are familiar to most people because we can see, feel and
even hear the spark as the excess charge is neutralized when
brought close to a large electrical conductor (for example a path to
ground), or a region with an excess charge of the opposite polarity
(positive or negative). The familiar phenomenon of a static 'shock'
is caused by the neutralization of charge.
The SI unit for measuring electric charge is the coulomb (C).
The symbol for charge is Q.
Physics is Life
How does Static Electricity
differ from Electric current?
Static electricity and electric current are two separate
phenomena, both involving electric charge, and may occur
simultaneously in the same object. Static electricity is a
reference to the electric charge of an object and the related
electrostatic discharge when two objects are brought
together that are not at equilibrium. An electrostatic
discharge creates a change in the charge of each of the two
objects. In contrast, electric current is the flow of electric
charge through an object, which produces no net loss or
gain of electric charge. Although charge flows between two
objects during an electrostatic discharge, time is too short
for current to be maintained.
Physics is Life
Introduction
A simple experiment will demonstrate the electrostatic phenomena.
Take a polythene rod and place one end of it near some
Experiment 1
pieces of paper.
Does anything happen?
Nothing will.
Physics is Life
Introduction
Rub the rod with a cloth and again place it near some pieces of paper as shown
in the diagram below.
Experiment 1
Does the rod affect the paper after being
rubbed?
When the rod is placed near the pieces
of paper,
some pieces of paper are attracted by
the “rubbed” polythene rod.
Physics is Life
Introduction
Experiment 1
This experiment tells us that the friction produced by rubbing the rod
must have affected the rod in some way. We can do further experiments
to discover the properties of such rods.
Physics is Life
Introduction
Further Electrostatics Experiments
Experiment 2
Observation:
The pith ball remains
unaffected even when the
uncharged glass rod is
placed very near to it.
Physics is Life
Introduction
Further Electrostatics Experiments
Experiment 2
Observation:
When the silk-rubbed glass rod is
placed near the pith ball, the ball
moves toward the rod.
Deduction:
The glass-rod is able to attract the pith ball
once it is rubbed with silk.
Physics is Life
Introduction
Further Electrostatics Experiments
Experiment 3
Observation:
When the fur-rubbed ebonite rod is
placed near the pith ball, the ball moves
toward the rod.
Deduction:
The ebonite-rod is able to attract the
pith ball
once it is rubbed with fur.
Physics is Life
Introduction
Further Electrostatics Experiments
Conclusion:
The glass & ebonite rods are said to be charged after
they are rubbed with silk & fur respectively.
Only charged rods are able to attract the pith ball.
Physics is Life
Introduction
Further Electrostatics Experiments
Observation:
Experiment 4
The angle displaced is less than the previous 2
angles in experiments 2 & 3.
Deduction:
The presence of the charged glass rod “weakens”
the “charged state” of the charged ebonite rod.
The presence of the charged ebonite rod “weakens”
the “charged state” of the charged glass rod.
Physics is Life
Introduction
Further Electrostatics Experiments
Experiment 5
Observation:
Repulsion occurs between 2 charged glass rods
Deduction:
All glass rods rubbed with silk are charged
similarly.
The charges in glass rods are thus identical
& like charges repel each other.
Physics is Life
Introduction
Further Electrostatics Experiments
Experiment 6
Observation:
Repulsion occurs between 2 charged
ebonite rods
Deduction:
ebonite
All ebonite rods rubbed with fur are
charged similarly.
The charges in fur rods are thus identical
& once again like charges repel each other.
Physics is Life
Introduction
Further Electrostatics Experiments
Experiment 7
Observation:
Attraction occurs between a charged ebonite
rod & a charged glass rod.
Deduction:
Charges in the ebonite rod & glass rod are
different.
Unlike charges attract each other.
Physics is Life
Introduction
Further Electrostatics Experiments
When you comb your hair and…
Experiment 8
… bring your comb over a pile of
paper bits
Physics is Life
Introduction
Further Electrostatics Experiments
Experiment 8
What will happen? {A or B}
A.
B.
Introduction
Further Electrostatics Experiments
Experiment 9
A charged object will also attract something that
is neutral. Think about how you can make a
balloon stick to the wall. If you charge a balloon
by rubbing it on your hair, it picks up extra
electrons and has a negative charge. Holding it
near a neutral object will make the charges in
that object move. If it is a conductor, many
electrons move easily to the other side, as far
from the balloon as possible. If it is an insulator,
the electrons in the atoms and molecules can
only move very slightly to one side, away from
the balloon. In either case, there are more
positive charges closer to the negative balloon.
Opposites attract. The balloon sticks. (At least
until the electrons on the balloon slowly leak
off.) It works the same way for neutral and
positively charged objects.
Physics is Life
Introduction
Further Electrostatics Experiments: Try this at Home!
Experiment 10
Light a light bulb with a balloon or rubber comb
You Need:
hard rubber comb or balloon
a dark room
fluorescent light bulb (not an incandescent bulb)
SAFETY NOTE: DO NOT USE ELECTRICITY FROM A WALL
OUTLET FOR THIS EXPERIMENT. Handle the glass light bulb with
care to avoid breakage. The bulb can be wrapped in sticky,
transparent tape to reduce the chance of injury if it does break.
What to do:
Take the light bulb and comb into the dark room.
Charge the comb on your hair or sweater. Make sure to build up a lot of charge for
this experiment.
Touch the charged part of the comb to the light bulb and watch very carefully. You
should be able to see small sparks. Experiment with touching different parts of the
bulb.
Physics is Life
Introduction
Actually, the thing we call static electricity is
an imbalance in the amounts of positive and
negative charges found on the surface of an
object.
Physics is Life
AIR SPARK
Rubbing action redistributes charge (unbalanced)
If enough charge builds up, we get discharge
Air spark is actually due to “breakdown” of air
– neutral air molecules separate into ions (electrons are stripped
away)
– current can then flow through the “plasma-field” air
– In essence, air becomes a “wire” for a short bit
– this happens at 3 million volts per meter
• 1 cm spark then at 30,000 volts
• typical finger-spark may involve a few billion electrons
Things you can do to reduce shock
Physics is Life
Lightning
Lightning is an unbelievably huge discharge
Clouds get charged through air friction
1 kilometer strike means 3 billion volts!
Main path forms temporary “wire” along which charge
equalizes
– often bounces a few times before equal
Thunder is the sound made by lightning. Depending on the
nature of the lightning and distance of the listener, it can
range from a sharp, loud crack to a long, low rumble
(brontide). The sudden increase in pressure and temperature
from lightning produces rapid expansion of the air
surrounding and within a bolt of lightning. In turn, this
expansion of air creates a sonic shock wave which produces
the sound of thunder.
Lightning strikes in the U.S.
More Information (Internet Link)
Physics is Life
Van de Graaff
electrostatic generator:
simulates lightning
from cloud to ground
How this Works
Physics is Life
Van de Graaf Generator Demonstrations
1.
2.
3.
4.
5.
6.
7.
Lightning/spark distance
Jumping balls in a box
Hair Raising
Deflect a Flame
Electric Wind
Blowing Bubbles
Encased in wire mesh
Physics is Life
Lightning Rods
Perform two functions
– provide safe conduit for lightning away from house
– diffuse situation via “coronal discharge”
Charges are attracted to tip of
rod, and
“electric field” is highly
concentrated
there.
Charges “leak” away, diffusing
charge
in what is sometimes
called “St. Elmo’sFire”, or
“coronal discharge”
Physics is Life
TRIBOELECTRIC SERIES
When we rub two different materials together, which
becomes positively charged and which becomes
negative? Scientists have ranked materials in order of
their ability to hold or give up electrons. This ranking
is called the triboelectric series. A list of some
common materials is shown here. Under ideal
conditions, if two materials are rubbed together, the
one higher on the list should give up electrons and
become positively charged. You can experiment with
things on this list for yourself .
Example#1: Rubbing rubber with fur makes
rubber negative and rabbit fur positive.
Example#2: Rubbing glass with silk makes
glass positive and silk negative.
Example#3: Rubbing your hand with scotch
tape makes the tape negative and your hand
Physics is Life
positive.
TRIBOELECTRIC SERIES
your hand
Rabbit fur
glass
your hair
nylon
wool
fur
silk
aluminum
paper
cotton
Wood
Amber
hard rubber
polyester
styrofoam
polyethylene (scotch tape)
teflon
TRIBOELECTRIC SERIES
If you take two pieces of tape-one
on top of another and rub them
with your hand, the tapes will be
negatively charged.
But if you were to separate the
tapes one strip of tape will be
negative and the other will be
positive.
What will happen to the charge if
you were to stick these tapes back
together?
Due to conservation of energy, the
net charge of the tapes will still be
negative.
Physics is Life
16.1 Static Electricity; Electric Charge
and Its Conservation
In conclusion, Charge comes in two
types, positive and negative; like
charges repel and opposite charges
attract.
Physics is Life
16.1 Static Electricity; Electric Charge
and Its Conservation
Benjamin Franklin (1706-1790) is credited for
naming the two types of charge. He argued
that whenever a certain charge is produced on
one body in a process, an equal amount of the
opposite type of charge is produced on
another body.The positive and negative
charges are to be treated algebraically, so that
during any process, the net change in the
amount of charge produced is zero.
This is an example of a law that is now well
established: the law of conservation of electric
charge, which states that the net amount of
charge produced in any process is zero.
Physics is Life
16.2 Electric Charge in the Atom
To understand electrostatics it is first important to
understand the basic structure of an atom.
An atom is made up of 3 different sub-atomic particles.
This is demonstrated in the following diagram showing an atom of beryllium.
-
proton
-
+
+
+
nucleus
nucleus
- electron
Physics is Life
+
neutron
16.2 Electric Charge in the Atom
Atom is electrically neutral. Sometimes however,
an atom may lose one or more of its electrons, or
may gain extra electrons. In this case the atom
will have a net positive or negative charge, and it
is called an ion.
In solid materials the nuclei tend to remain to
fixed positions where as some of the electrons
move quite freely. The charging of a solid object
by rubbing is explained mainly by the transfer of
electrons from one material to another.
The electric force between the electrons and
protons supplies the centripetal force to keep
electrons in the atom.
We will discuss the equation for the electric
force in detail later in this presentation.
Physics is Life
16.2 Electric Charge in the Atom
Polar molecule: neutral overall, but charge not evenly
distributed
Normally when objects are charged by rubbing, they hold their electrons
only for a limited time and eventually return to the neutral state. The excess
charges may be ‘leaked off” onto water molecules in the air. What do you
think is going to happen if you bring a charged rubber rod to a steady
stream of water?
Physics is Life
16.3 Insulators and Conductors
The electrons moving around the nucleus can be moved from
an atom to another atom, and from object to object. These
electrons will move depending on whether the material is a
conductor or an insulator
Some of the electrons in a conductor are held loosely by the atom.
Such electrons move freely from atom to atom within the material.
(Example: Metals)
In insulators, the electrons are held tightly to the atom and are not
able to move freely within the material. (example: Wood, fur, glass,
etc.)
Physics is Life
16.3 Insulators and Conductors
Insulators
Materials that do not allow electrons to move freely inside them are called electrical
insulators.
An electrical insulator has electrons that are all in fixed positions.
The addition or removal of electrons at any one part of the insulator does not
result in the electrons in other parts of the same insulator to move.
Thus, we say that the charge is localised (or confined) to the region.
Physics is Life
16.3 Insulators and Conductors
Insulators keep electricity from leaving power lines. Glass, plastic, or
ceramic insulators high up on power poles keep electricity from
traveling down the pole to the ground. If an insulator breaks, or a power
line becomes disconnected from the insulators that hold it up, the line
can fall to the ground and energize the area around it with a lot of
electricity. If you touch a downed line — or even the ground near the
line — you could be hurt or killed. If a power line falls on a car and you
touch the car and the ground at the same time, you would also get a
shock.
Examples of insulators are wood, plastics, ebonite, glass, fur,
silk.
The method of charging by friction will only work when two
insulators are rubbed against each other. When an insulator is
charged by the friction method the charge remains on the
surface of the material. This is because the charge cannot
move through the insulator.
Physics is Life
16.3 Insulators and Conductors
Insulators
A positively-charged insulator can be discharged (to lose all its charges)
by passing it quickly over a flame.
The air above a flame consists of many ions (both positive & negative).
When a positively-charged insulator (excess positive charge)
passes over a flame,
the negatively-ions will be attracted to the positive charges in the insulator.
This causes the positive charges to be neutralized by the negative ions.
+
-
-
+
+
+
+
-
+
+
-
+
-
Physics is Life
+
16.3 Insulators and Conductors
Insulators
A positively-charged insulator can be discharged (to lose all its charges)
by passing it quickly over a flame.
The air above a flame consists of many ions (both positive & negative).
When a positively-charged insulator (excess positive charge)
passes over a flame,
the negatively-ions will be attracted to the positive charges in the insulator.
This causes the positive charges to be neutralized by the negative ions.
+
-
-
+
+
+
+
-
+
+
-
+
-
Physics is Life
+
16.3 Insulators and Conductors
Insulators
A positively-charged insulator can be discharged (to lose all its charges)
by passing it quickly over a flame.
The air above a flame consists of many ions (both positive & negative).
When a positively-charged insulator (excess positive charge)
passes over a flame,
the negatively-ions will be attracted to the positive charges in the insulator.
This causes the positive charges to be neutralized by the negative ions.
-
+
+
-
+
+
+
-
Physics is Life
+
16.3 Insulators and Conductors
Discharging Insulators
Summarising, all charged insulators can be discharged by passing them over a flame.
Ions present in the air above the flame will be attracted towards the charges present in
the charged insulators.
These ions will neutralize the charges in the insulators, thus discharging them.
Physics is Life
16.3 Insulators and Conductors
Conductors
Some materials allow electrons to move about easily inside them.
These are called electrical conductors.
-
-
-
All metals are conductors of electricity.
All conductors can be discharged easily by a method known as
Grounding) .
Physics is Life
42
16.3 Insulators and Conductors
Conductors
In electrical conductors, the outer electrons (also known as valence electrons) are loosely
bound. They are relatively free from individual atoms.
We say that these electrons are
delocalized.
When electrons are gained by the
conductors, the other electrons
will flow automatically so that
electron re-distribution in the
conductors occur.
When electrons are lost by the
conductors, the other electrons
will also flow automatically so
that electron re-distribution in the
conductors occur.
Physics is Life
16.3 Semi conductors vs. Super
conductors
Semiconductors are materials which are good
insulators in pure form, but their conducting
properties can be adjusted over a wide range
by introducing small amounts of impurities.
Examples are silicon and germanium
Superconductors are materials that lose all
resistance to charge movement at
temperatures near absolute zero (0 K or about
-273 C)
Recently, “high temperature” (Above 100 K)
superconductors have been discovered.
Physics is Life
16.4 Induced Charge
Metal objects can be charged by conduction:
Physics is Life
16.4 Induced Charge
They can also be charged by induction:
Physics is Life
16.4 Induced Charge
Nonconductors won’t become charged by
conduction or induction, but will experience
charge separation:
Physics is Life
16.4 Induced Charge
Bringing a charged object near (but not
touching) a neutral object polarizes
(temporarily separates) the charge of the
neutral object.
Like charges in the neutral object are
repelled by the charged object.
Unlike charges in the neutral object are
attracted by the charged object.
The neutral object returns to normal when
the charged object is removed.
An object that is electrically neutral
overall, but permanently polarized, is
called an electric dipole. An example is
the water molecule.
Physics is Life
Click here for simulation
16.4 Induced Charge; the Electroscope
The electroscope
can be used for
detecting charge:
Physics is Life
16.4 Induced Charge; the Electroscope
The
electroscope can
be charged
either by (a)
induction or by
(b) conduction.
Physics is Life
16.4 Induced Charge; the Electroscope
The charged electroscope can then be used to
determine the sign of an unknown charge.
Physics is Life
16.5 Coulomb’s Law
Experiment shows that
the electric force
between two charges is
proportional to the
product of the charges
and inversely
proportional to the
distance between them.
Physics is Life
16.5 Coulomb’s Law
Coulomb’s law:
(16-1)
Where Q1 and Q2 are the amount of charge and k
is a proportionality constant
Charges produced by rubbing ordinary objects
(such as a comb or a plastic ruler) are typically
around a microcoulomb or less:
Physics is Life
16.5 Coulomb’s Law
The magnitude of the charge of an electron, on the other
hand, has been determined to be about 1.602 x 10-19 C, and
its sign is negative. This is the smallest known charge, and
because of its fundamental nature, it is given the symbol e
and is often referred to as the elementary charge:
Example:
How many electrons make up a charge of -30.0 micro
coulombs (C)?
N = Q/e = (-30 x 10-6 C)/ (-1.60 x 10-19 C/electrons) = 1.88 x 1014 electrons
What is the mass of 1.88 x 1014 electrons?
Mass = (9.11 x 10-31 kg)(1.88 x 1014 electrons) = 1.71 x 10-16 kg
Physics is Life
54
16.5 Coulomb’s Law
The charges carried by the proton and
electron are equal in size. However, the
mass of the proton is 2000 times the
mass of the electron.
Physics is Life
16.5 Coulomb’s Law
Double one of the charges
– force doubles
Change sign of one of the charges
– force changes direction
Change sign of both charges
– force stays the same
Double the distance between charges
– force four times weaker
Double both charges
– force four times stronger
Physics is Life
Coulomb’s Law vs. Law of
Universal Gravitation
F = kQ1Q2/r2 vs. F=GM1M2/r2
Both are inverse square laws F1/r2
Both have a proportionality to a product of each body-
mass for gravity, electric charge for electricity.
A major difference is that gravity is always an attractive
force, whereas the electric force can be wither attractive or
repulsive.
Electrical Force is stronger than gravitational force
Comparison of electrical force vs. Gravitational force #1
Comparison of electrical force vs. Gravitational Force #2
Physics is Life
16.6 Solving Problems involving
Coulomb’s Law
Sample problem
Find the force between two positive 1.0 C charges
when they are 1000m apart?
Solution
q1=q2 = 1.0C
r = 1000m
F = kq1q2/r2 where k = 9.0 x 109 Nm2/C2
After substitution, F = 9.0 x 103 N
Physics is Life
58
16.6 Solving Problems involving
Coulomb’s Law
Sample problem
What is the magnitude of the electric force of
attraction between an iron nucleus (q = +26e) and
its innermost electron if the distance between them
is 1.5 x 10-12 m?
Solution
F = kq1q2/r2 where k = 9.0 x 109 Nm2/C2
F = (9.0 x 109 Nm2/C2)(26)(1.6 x 10-19 C)(1.6 x 10-19 C)/
(1.5 x 10-12 m)2 = 2.7 x 10-3 N
Physics is Life
16.6 Solving Problems involving
Coulomb’s Law
Sample problem
What is the repulsive electrical force between two
protons in a nucleus that are 5.0 x 10-15 m apart
from each other?
Solution
F = kq1q2/r2 where k = 9.0 x 109 Nm2/C2
F = (9.0 x 109 Nm2/C2)(1.6 x 10-19 C)(1.6 x 10-19 C)/
(5.0 x 10-15 m)2 = 9.2 N
Physics is Life
16.6 Solving Problems involving
Coulomb’s Law
Sample problem
Two charged balls are 20.0 cm apart. They are
moved, and the force on each of them is found to
have been tripled. How far apart are they now?
Solution
Let F1 = kq1q2/r12 and F2 = kq1q2/r22 where F2 = 3 F1
F2/F1 = r12 /r22
3= [(20.0cm)/r2]2, which gives r2 = 11.5 cm
Physics is Life
16.7 The Electric Field
In physics, the space surrounding an
electric charge has a property called an
electric field. This electric field exerts a
force on other electrically charged
objects. The concept of an electric field
was introduced by Michael Faraday.
The electric field is a vector field with SI
units of newtons per coulomb (N C−1) or,
equivalently, volts per meter. The strength
of the field at a given point is defined as
the force that would be exerted on a
positive test charge of +1 coulomb placed
at that point; the direction of the field is
given by the direction of that force.
Michael Faraday (1791-1867)
Physics is Life
62
16.7 The Electric Field
Measuring an electric field is a quite simple process involving a
test charge. To measure the strength of an electric field, first a
test charge must be placed in its vicinity, then calculate the force
the test charge “feels”. The resulting number is the strength of
the electric field. This process is simplified into the following
equation
(16-3)
In this equation,F is the magnitude of the force, as found by using
Coulomb's Law, q is the magnitude of the test charge. The
resulting electric strength is measured in Newton’s per a
Coulomb.
Physics is Life
63
16.7 The Electric Field
One can think of electric force as establishing a “field” telling particles
which way to move and how fast
Electric “field lines” tell a positive
charge which way to move.
For example, a positive charge itself
has field lines pointing away from it,
because this is how a positively-charged
“test-particle” would respond if placed
in the vicinity (repulsive force).
+
Run Away!
+
Physics is Life
64
16.7 The Electric Field
The direction of the
electric field is
always directed in
the direction that a
positive test charge
would be pushed or
pulled if placed in the
space surrounding
the source charge
+
+
+
Physics is Life
+
65
Electric Field vs. Gravitational Field
Right now you are experiencing a uniform gravitational field: it has a magnitude of 9.8 m/s 2 and
points straight down. If you threw a mass through the air, you know it would follow a parabolic
path because of gravity. You could determine when and where the object would land by doing a
projectile motion analysis, separating everything into x and y components. The horizontal
acceleration is zero, and the vertical acceleration is g. We know this because a free-body
diagram shows only mg, acting vertically, and applying Newton's second law tells us that mg =
ma, so a = g.
You can do the same thing with charges in a uniform electric field. If you throw a charge into a
uniform electric field (same magnitude and direction everywhere), it would also follow a
parabolic path. We're going to neglect gravity; the parabola comes from the constant force
experienced by the charge in the electric field. Again, you could determine when and where the
charge would land by doing a projectile motion analysis. The acceleration is again zero in one
direction and constant in the other. The value of the acceleration can be found by drawing a
free-body diagram (one force, F = qE) and applying Newton's second law. This says: qE = ma,
so the acceleration is a = qE / m.
The one big difference between gravity and electricity is that m, the mass, is always positive,
while q, the charge, can be positive, zero, or negative.
66
16.7 The Electric Field
Sample Problem
A positive charge of 1.0 x 10-5C experiences a
force of 0.30N when located at a certain point.
What is the electric field intensity at that
point?
Solution
E=F/Q = 0.30N / 1.0 x 10-5 C = 3.0 x 104 N/C
Physics is Life
67
16.7 The Electric Field
Sample Problem
A test charge experiences a force of 0.20 N on
it when it is placed in an electric field intensity
of 4.5 x 105 N/C. What is the magnitude of the
charge?
Solution
Q=F/E = 0.20N / 4.5 x 105 N/C = 4.4 x 10-7 C
Physics is Life
16.7 The Electric Field
Sample Problem
A positive charge of 10-5 C experiences a
force of 0.2N when located at a certain point
in an electric field. What is the electric field
strength at that point?
Solution
F= 0.2N
q=10-5C
E= F/q = 0.2N/10-5C = 2 x 104 N/C
Physics is Life
16.7 The Electric Field
Using E = F/Q and substitute for F
using Coulumb’s Law, The electric
field for a point charge can be
rewritten as:
(16-4a)
The magnitude or strength of an electric field in the space
surrounding a source charge is related directly to the quantity of
charge on the source charge and inversely to the distance from the
source charge.
Physics is Life
70
16.7 The Electric Field
Sample Problem
Calculate the magnitude and direction of the electric
field at a point P which is 30cm to the right of a
point charge Q= -3.0x 10-6C.
Solution
E=kQ/r2 = (9 x 109 Nm2/C2)(3 x 10-6C) / (0.30m)2 = 3.0 x 105N/C
The direction of the electric field is toward the charge Q since we
defined the direction as that of the force on a positive test
charge.
Physics is Life
16.8 Field Lines
The electric field can be represented by field lines.
These lines start on a positive charge and end on a
negative charge.
At locations where electric field lines meet the
surface of an object, the lines are perpendicular
to the surface.
Physics is Life
72
16.8 Field Lines
Electric dipole: two equal charges, opposite in
sign:
•Field lines indicate the
direction of the field; the
field is tangent to the line.
Physics is Life
73
16.8 Field Lines
The number of field lines
starting (ending) on a
positive (negative) charge
is proportional to the
magnitude of the charge.
Electric field lines never
cross each other.
The electric field is stronger
where the field lines are
closer together.
Physics is Life
74
16.8 Field Lines
The electric field between two
closely spaced, oppositely charged
parallel plates is constant.
Physics is Life
16.8 Field Lines
Summary of Field lines Around Charges
1. The magnitude of the field is proportional to the density of the
lines.
2. Field lines start on positive charges and end on negative charges;
the number is proportional to the magnitude of the charge.
3. Field lines indicate the direction of the field; the field is tangent to
the line.
•
The electric field between two closely spaced, oppositely charged
parallel plates is constant.
•
At locations where electric field lines meet the surface of an object,
the lines are perpendicular to the surface.
•
Electric field lines never cross each other.
Physics is Life
76
Millikan’s Oil Drop Experiment
One important application of the uniform electric
field between two parallel plates is the measurement
of charge of an electron. This was determined by
American physicist Robert A Millikan in 1909
Millikan’s experiment showed that charge is quantized. This
means that an object can only have a charge with a
magnitude that is some integral multiple of the charge of the
electron (1.6 x 10-19 C). Physics is Life
Millikan’s Oil Drop Experiment
Sample Problem
In a Millikan oil drop experiment, a drop has been found to
weigh 1.9 x 10-14 N. When the electric field is 4.0 x 104 N/C,
the drop is suspended motionless. (a) what is the charge on
the drop? (b) If the upper plate is positive, how many excess
electrons does the oil drop have?
Solution
(a) When balanced, Felectric = F gravity Thus, qE=mg solving for q, the charge
will be
q=mg/E = 1.9 x 10-14 N/4.0 x 104N/C = 4.8 x 10-19 C
(b) Determine the number of electrons by n=q/e
n=4.4 x 10-19 C/1.6 x 10-19 C/electron = 3 electrons
Physics is Life
Millikan’s Oil Drop Experiment
Sample Problem
A positively charged oil drop weighs 6.4 x 10-13 N. An electric
field of 4.0 x 106 N/C suspends the drop. (a) What is the charge
on the drop? (b) How many electrons is the drop missing?
Solution
(a) Q=F/E = 6.4 x 10-13 N/ 4.5 x 106 N/C = 1.6 x 10-19
C
(b) N = Q/e = 1.6 x 10-19 C/1.6 x 10-19 C/electron =
1 electron
Physics is Life
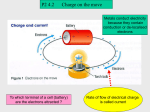
16.9 Electric Fields and Conductors
We have previously shown that any charged object - positive or
negative, conductor or insulator - creates an electric field which
permeates the space surrounding it. In the case of conductors there
are a variety of unusual characteristics about which we could
elaborate. Recall that a conductor is material which allows electrons
to move relatively freely from atom to atom. It was emphasized that
when a conductor acquires an excess charge, the excess charge
moves about and distributes itself about the conductor in such a
manner as to reduce the total amount of repulsive forces within the
conductor. Electrostatic equilibrium is the condition established by
charged conductors in which the excess charge has optimally
distanced itself so as to reduce the total amount of repulsive forces.
Once a charged conductor has reached the state of electrostatic
equilibrium, there is no further motion of charge about the surface.
Physics is Life
16.9 Electric Fields and Conductors
Charged conductors which have reached electrostatic equilibrium share
three particular characteristics. One characteristic of a conductor at
electrostatic equilibrium is that the electric field anywhere beneath the
surface of a charged conductor is zero. If an electric field did exist beneath
the surface of a conductor (and inside of it), then the electric field would
exert a force on all electrons that were present there. This net force would
begin to accelerate and move these electrons. But objects at electrostatic
equilibrium have no further motion of charge about the surface. So if this
were to occur, then the original claim that the object was at electrostatic
equilibrium would be a false claim. If the electrons within a conductor have
assumed an equilibrium state, then the net force upon those electrons is
zero. The electric field lines either begin or end upon a charge and in the
case of a conductor, the charge exists solely upon its outer surface. The
lines extend from this surface outward, not inward.
Physics is Life
16.9 Electric Fields and Conductors
This concept of the electric field being zero inside of a closed
conducting surface was first demonstrated by Michael Faraday. Faraday
constructed a room within a room, covering the inner room with a metal foil.
He sat inside the inner room with an electroscope and charged the surfaces of
the outer and inner room using an electrostatic generator. While sparks were
seen flying between the walls of the two rooms, there was no detection of an
electric field within the inner room. The excess charge on the walls of the
inner room resided entirely upon the outer surface of the room.
The inner room with the conducting frame which protected Faraday
from the static charge is now referred to as a Faraday's cage. The cage serves
to shield whomever and whatever is on the inside from the influence of
electric fields. Any closed, conducting surface can serve as a Faraday's cage,
shielding whatever it surrounds from the potentially damaging affects of
electric fields. This principle of shielding is commonly utilized today as we
protect delicate electrical equipment by enclosing them in metal cases. Even
delicate computer chips and other components are shipped inside of
conducting plastic packaging which shields the chips from potentially
damaging affects of electric fields.
Physics is Life
16.9 Electric Fields and Conductors
The excess charges arrange themselves in a the
conductor surface precisely in the manner needed to
make the electric field zero within the material.
Physics is Life
83
16.9 Electric Fields and Conductors
We can also see
that a relatively
safe place to be
during a
lightning storm is
inside a car,
surrounded by
metal..
Physics is Life
84
16.9 Electric Fields and Conductors
A second characteristic of conductors at electrostatic equilibrium is
that the electric field upon the surface of the conductor is directed
entirely perpendicular to the surface. There cannot be a component of
electric field (or electric force) that is parallel to the surface. If the
conducting object is spherical, then this means that the perpendicular
electric field vector are aligned with the center of the sphere. If the
object is irregularly shaped, then the electric field vector at any location
is perpendicular to a tangent line drawn to the surface at that location.
Physics is Life
16.9 Electric Fields and Conductors
A third characteristic of conducting objects at electrostatic equilibrium is that
the electric fields are strongest at locations along the surface where the object is
most curved. The curvature of a surface can range from absolute flatness on one
extreme to being curved to a blunt point on the other extreme. A flat location has
no curvature and is characterized by relatively weak electric fields. On the other
hand, a blunt point has a high degree of curvature and is characterized by
relatively strong electric fields. A sphere is uniformly shaped with the same
curvature at every location along its surface. As such, the electric field strength
on the surface of a sphere is everywhere the same. But on an irregularly shaped
object, excess electrons would tend to accumulate in greater density along
locations of greatest curvature.
Physics is Life
16.9 Electric Fields and Conductors
Summary of Field lines Around Conductors
• The electric field anywhere beneath the surface of a charged
conductor in static equilibrium is zero; excess charge of a
conductor exists solely on its surface.
• The electric field of the surface of the conductor at
electrostatic equilibrium is directed entirely perpendicular to
the surface.
• Conductors at electrostatic equilibrium exert strong electric
fields along any curvature or sharp bend at its surface.
Physics is Life
Summary of Chapter 16
• Two kinds of electric charge – positive and
negative
• Charge is conserved
• Charge on electron:
• Conductors: electrons free to move
• Insulators: nonconductors
Physics is Life
Summary of Chapter 16
• Charge is quantized in units of e
• Objects can be charged by conduction or induction
• Coulomb’s law:
• Electric field is force per unit charge:
•Electric field of a point charge:
Physics is Life
89
Summary of Chapter 16
http://www.stmary.ws/highschool/physics/home/not
es/electricity/staticElectricity/default.htm
MIT LECTURE ON ELECTRIC FIELD
• Electric field can be represented by electric field lines
• Static electric field inside conductor is zero; surface Efield is perpendicular to surface; Higher density of field
lines signify a stronger E-field.
Physics is Life
90