* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Item VIII
Relativistic quantum mechanics wikipedia , lookup
Matter wave wikipedia , lookup
Wave–particle duality wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Quantum state wikipedia , lookup
Particle in a box wikipedia , lookup
Ising model wikipedia , lookup
Density matrix wikipedia , lookup
Copenhagen interpretation wikipedia , lookup
Interpretations of quantum mechanics wikipedia , lookup
Hidden variable theory wikipedia , lookup
Probability amplitude wikipedia , lookup
Renormalization group wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Path integral formulation wikipedia , lookup
Canonical quantization wikipedia , lookup
Supplement
– Statistical Thermodynamics
고려대학교 화공생명공학과
강정원
Contents
1. Introduction
2. Distribution of Molecular States
3. Interacting Systems – Gibbs Ensemble
4. Classical Statistical Mechanics
1. Introduction
Mechanics : Study of position, velocity, force and
energy
Classical Mechanics (Molecular Mechanics)
• Molecules (or molecular segments) are treated as rigid object
(point, sphere, cube,...)
• Newton’s law of motion
Quantum Mechanics
• Molecules are composed of electrons, nuclei, ...
• Schrodinger’s equation Wave function
1. Introduction
Methodology of Thermodynamics and Statistical Mechanics
Thermodynamics
• study of the relationships between macroscopic properties
–
Statistical Mechanics (Statistical Thermodynamics)
•
how the various macroscopic properties arise as a consequence of the microscopic nature of
the system
–
Volume, pressure, compressibility, …
Position and momenta of individual molecules (mechanical variables)
Statistical Thermodynamics (or Statistical Mechanics) is a link between microscopic
properties and bulk (macroscopic) properties
Thermodynamic Variables
Pure mechanical variables
Statistical
Mechanics
Methods of QM
Methods of MM
U
A particular
microscopic model
can be used
r
P
T
V
1.Introduction
Equilibrium Macroscopic Properties
Properties are consequence of average of individual
molecules
Properties are invariant with time Time average
Mechanical
Properties of
Individual
Molecules
position, velocity
energy, ...
average
over molecules
2
average
over time
3
statistical
thermodynamics
4
Thermodynamic
Properties
temperature, pressure
internal energy, enthalpy,...
1. Introduction
Description of States
Macrostates : T, P, V, … (fewer variables)
Microstates : position, momentum of each particles (~1023 variables)
Fundamental methodology of statistical mechanics
Probabilistic approach : statistical average
• Most probable value
Is it reasonable ?
• As N approaches very large number, then fluctuations are negligible
• “Central Limit Theorem” (from statistics)
• Deviation ~1/N0.5
2. Distribution of Molecular States
Statistical Distribution
n : number of occurrences
b : a property
if we know “distribution”
we can calculate the average
value of the property b
ni
b
1 2 3 4 5 6
2. Distribution of Molecular States
Normalized Distribution Function
Probability Distribution Function
Pi (bi )
ni (bi )
n (b )
i i
n
ni (bi )
i
P (b ) 1
i
i
i
Pi
b bi Pi
i
b1 b2 b3 b4 b5 b6
b
F (b) F (bi ) Pi
i
Finding probability (distribution) function is
the main task in statistical thermodynamics
2. Distribution of Molecular States
Quantum theory says ,
Each molecules can have only discrete values of energies
Evidence
Black-body radiation
Planck distribution
Heat capacities
Atomic and molecular spectra
Wave-Particle duality
Energy
Levels
2. Distribution of Molecular States
Configuration ....
At any instance, there may be no molecules at e0 , n1
molecules at e1 , n2 molecules at e2 , …
{n0 , n1 , n2 …} configuration
e
5
e4
e3
e2
e
1e
0
{ 3,2,2,1,0,0}
2. Distribution of Molecular States
Weight ....
Each configurations can be achieved in different ways
Example1 : {3,0} configuration 1
e
1e
0
Example2 : {2,1} configuration 3
e
1e
e
1e
e
1e
0
0
0
2. Distribution of Molecular States
Calculation of Weight ....
Weight (W) : number of ways that a configuration can be achieved in
different ways
General formula for the weight of {n0 , n1 , n2 …} configuration
N!
N!
W
n1!n2 !n3!... ni !
Example1
{1,0,3,5,10,1} of 20 objects
W = 9.31E8
i
Example 2
{0,1,5,0,8,0,3,2,1} of 20 objects
W = 4.19 E10
Principles of Equal a Priori Probability
All distributions of energy are equally probable
If E = 5 and N = 5 then
5
5
5
4
3
2
1
0
4
3
2
1
0
4
3
2
1
0
All configurations have equal probability, but
possible number of way (weight) is different.
A Dominating Configuration
For large number of molecules and large number of energy
levels, there is a dominating configuration.
The weight of the dominating configuration is much more
larger than the other configurations.
Wi
Configurations
{ni}
Dominating Configuration
W = 1 (5!/5!)
5
5
5
4
3
2
1
0
4
3
2
1
0
4
3
2
1
0
W = 20 (5!/3!)
W = 5 (5!/4!)
Difference in W becomes larger when N is increased !
In molecular systems (N~1023) considering the
most dominant configuration is enough for average
How to find most dominant
configuration ?
The Boltzmann Distribution
Task : Find the dominant configuration for given N and
total energy E
Method : Find maximum value of W which satisfies,
N ni
i
E e i ni
i
dn
i
0
i
e dn
i
i
i
0
Stirling's approximation
A useful formula when dealing with factorials of
large numbers.
ln N! N ln N N
N!
ln W ln
ln N ! ln ni !
n1!n2 !n3!...
i
N ln N N ni ln ni ni
i
N ln N ni ln ni
i
i
Method of Undetermined
Multipliers
Maximum weight , W
Recall the method to find min, max of a function…
d ln W 0
ln W
0
dni
Method of undetermined multiplier :
Constraints should be multiplied by a constant and
added to the main variation equation.
Method of Undetermined
Multipliers
undetermined multipliers
ln W
dni dni e i dni
d ln W
i dni
i
i
ln W
ei dni 0
i dni
ln W
ei 0
dni
Method of Undetermined
Multipliers
ln W N ln N ni ln ni
ln W
ni
(n j ln n j )
N ln N
ni
ni
j
N ln N N
1 N
ln N N
ln N 1
ni
N ni
ni
j
(n j ln n j )
ni
n j
1
ln n j n j
nj
j
ni
n
ln W
(ln ni 1) (ln N 1) ln i
ni
N
n j
ln ni 1
ni
Method of Undetermined
Multipliers
ln
ni
ei 0
N
ni
e e i
N
Normalization Condition
N n j Ne e
j
e
e j
j
1
e
e j
j
ni
e e i
Pi
N e e j
j
Boltzmann Distribution
(Probability function for
energy distribution)
The Molecular Partition Function
Boltzmann Distribution
ni
e e i
e e i
pi
e j
N e
q
j
Molecular Partition Function
q e
e j
j
Degeneracies : Same energy value but different states (gjfold degenerate)
q
g je
levels
j
e j
How to obtain the value of beta ?
Assumption :
1 / kT
T 0 then q 1
T infinity then q infinity
The molecular partition function gives an indication of the
average number of states that are thermally accessible to a
molecule at T.
2. Interacting Systems
– Gibbs Ensemble
Solution to Schrodinger equation (Eigen-value problem)
Wave function
Allowed energy levels : En
h2
2 i2 U E
i 8 mi
Using the molecular partition function, we can calculate
average values of property at given QUANTUM STATE.
Quantum states are changing so rapidly that the observed
dynamic properties are actually time average over quantum
states.
Fluctuation with Time
states
time
Although we know most probable distribution of energies of individual
molecules at given N and E (previous section – molecular partition
function) it is almost impossible to get time average for interacting
molecules
Thermodynamic Properties
Entire set of possible quantum states
1 , 1 , 1 ,...i ,...
E1 , E2 , E3 ,..., Ei ,...
Thermodynamic internal energy
U lim
1
E t
i
i
i
Difficulties
Fluctuations are very small
Fluctuations occur too rapidly
We have to use alternative, abstract approach.
Ensemble average method (proposed by Gibbs)
Alternative Procedure
Canonical Ensemble
Proposed by J. W. Gibbs (1839-1903)
Alternative procedure to obtain average
Ensemble : Infinite number of mental replica
of system of interest
Large reservoir (constant T)
All the ensemble members have the same (n, V, T)
Energy can be exchanged but
particles cannot
Number of Systems : N
N∞
Two Postulate
Fist Postulate
The long time average of a mechanical variable M is
equal to the ensemble average in the limit N ∞
time
E1
E2
E3
E4
E5
Second Postulate (Ergodic Hypothesis)
The systems of ensemble are distributed uniformly for (n,V,T) system
Single isolated system spend equal amount of time
Averaging Method
Probability of observing particular quantum state i
n~i
Pi
n~i
i
Ensemble average of a dynamic property
E Ei Pi
i
Time average and ensemble average
U lim Ei ti lim
n
E P
i i
i
How to find Most Probable
Distribution ?
Calculation of Probability in an Ensemble
Weight
~
~
N!
N!
W ~ ~ ~
n1!n2 !n3!... n~i !
i
Most probable distribution = configuration with maximum weight
Task : find the dominating configuration for given N and E
• Find maximum W which satisfies
~
N n~i
i
Et Ei n~i
i
~ 0
d
n
i
i
~ 0
E
d
n
i i
i
Canonical Partition Function
Similar method (Section 2) can be used to get most
probable distribution
ni
e Ei
Pi
N e E j
j
ni
e Ei
e Ei
Pi
E j
N e
Q
j
Q e
j
E j
Canonical Partition Function
How to obtain beta ?
– Another interpretation
dU d ( Ei Pi ) Ei dPi Pi dEi
i
i
i
dU qrev wrev TdS pdV
Ei
P dE P V
i
i
i
i
Ei dPi
i
dV PdV wrev
i
1
N
( ln Pi dPi ln Q dPi )
i
i
1
ln P dP TdS dq
i
i
i
The only function that links heat (path integral) and
state property is TEMPERATURE.
1 / kT
rev
Properties from Canonical Partition
Function
Internal Energy
U E Ei Pi
i
1
Ei
E
e
i
Q i ( qs)
Q
Ei e Ei
i ( qs )
N ,V
ln Q
1 Q
U
Q N ,V
N ,V
Properties from Canonical Partition
Function
Pressure
(wi ) N Pi dV Fi dx
Small Adiabatic expansion of system
(dEi ) N Fi dx Pi dV wi
dx
E
Pi i
V N
P P Pi Pi
Fi
dV
V
i
P
1
1
Ei Ei
Ei
P
e
e
i
Q i
Q i V N
E
ln Q
i e Ei
V , N Q i V N
P
1 ln Q
ln V
dEi
Ei
Thermodynamic Properties from
Canonical Partition Function
ln Q
)V , N
ln T
ln Q
S k ln Q (
)V , N
ln T
U kT (
ln Q
ln Q
H kT (
)V , N (
)T , N
ln V
ln T
A kT ln Q
ln Q
G kT ln Q (
)T , N
ln V
ln Q
i kT
N i T ,V , N
j i
Grand Canonical Ensemble
Ensemble approach for open system
Useful for open systems and mixtures
Walls are replaced by permeable walls
Large reservoir (constant T )
All the ensemble members have the same (V, T, i )
Energy and particles can be exchanged
Number of Systems : N
N∞
Grand Canonical Ensemble
Similar approach as Canonical Ensemble
We cannot use second postulate because systems are not isolated
After equilibrium is reached, we place walls around ensemble and treat
each members the same method used in canonical ensemble
After
equilibrium
T,V,
T,V,N1
T,V,N3
T,V,N2
Each members are (T,V,N) systems
Apply canonical ensemble methods for each member
T,V,N5
T,V,N4
Grand Canonical Ensemble
Weight and Constraint
n j ( N )!
j,N
W
n j ( N )!
Number of ensemble members
N n j (N )
Number of molecules after
fixed wall has been placed
j,N
Et n j ( N ) E j (V , N )
j,N
j,N
Nt n j ( N ) N
Method of undetermined multiplier
with , ,g
n*j ( N ) Ne e
Pj ( N )
j,N
E j ( N ,V ) gN
n j (N )
N
e
n*j ( N )
N
e
E j ( N ,V ) gN
e
j,N
e
E j ( N ,V ) gN
e
Grand Canonical Ensemble
Determination of Undetermined Multipliers
U E Pj ( N ) E j ( N ,V )
e
j,N
dU E j ( N ,V )dPj ( N ) Pj ( N )dE j ( N ,V )
j,N
dU
E j ( N ,V ) gN
j,N
j,N
E ( N ,V )
g
N
ln
P
(
N
)
ln
dP
(
N
)
P
(
N
)
dV
V
1
j
j
j
j
j,N
j,N
dU TdS pdV dN
Comparing two equation gives,
e
j,N
E j ( N ,V ) / kT
e
N / kT
1
kT
g
kT
Grand Canonical Partition Function
e
4. Classical Statistical Mechanics
The formalism of statistical mechanics relies very much at the
microscopic states.
Number of states , sum over states
convenient for the framework of quantum mechanics
What about “Classical Sates” ?
Classical states
• We know position and velocity of all particles in the system
Comparison between Quantum Mechanics and Classical Mechanics
QM Problem
H E
Finding probability and discrete energy states
CM Problem
F ma
Finding position and momentum of individual molecules
Newton’s Law of Motion
Three formulations for Newton’s second law of motion
Newtonian formulation
Lagrangian formulation
Hamiltonian formulation
H (r N , p N ) KE(kinetic energy) PE(potenti al energy)
p
H (r N , p N ) i U (r1 , r2 ,..., rN )
i 2mi
H
p i
ri
H
ri
p i
ri p i
t mi
r r (rx , ry , rz )
p i
Fi
t
Fi Fij
p p( p x , p y , p z )
j 1
j i
Classical Statistical Mechanics
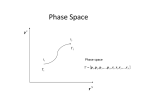
Instead of taking replica of systems, use abstract “phase space”
composed of momentum space and position space (total 6N-space)
pN
t2
2
t1
1
Phase space
p1 , p 2 , p3 ,..., p N , r1 , r1 , r3 ,..., rN
rN
Classical Statistical Mechanics
“ Classical State “ : defines a cell in the space (small volume of
momentum and positions)
" Classical State" dqx dq y dqz drx dry drz d 3 pd 3r for simplicity
Ensemble Average
U lim
1
0
E ()d lim P N () E ()d
PN ()d
n
Fraction of Ensemble members in this range
( to +d)
Using similar technique used for
Boltzmann distribution
PN ()d
exp( H / kT )d
... exp( H / kT )d
Classical Statistical Mechanics
Canonical Partition Function
Phase Integral
T ... exp( H / kT )d
Canonical Partition Function
Q c ... exp( H / kT )d
Match between Quantum
and Classical Mechanics
c lim
T
exp( E / kT )
i
i
... exp( H / kT )d
1
N!h NF
For rigorous derivation see Hill, Chap.6 (“Statistical Thermodynamics”)
c
Classical Statistical Mechanics
Canonical Partition Function in Classical Mechanics
1
Q
... exp( H / kT )d
NF
N !h
Example ) Translational Motion for
Ideal Gas
H (r N , p N ) KE(kinetic energy) PE(potenti al energy)
H (r N , p N )
i
3N
H
i
pi
U (r1 , r2 ,..., rN )
2mi
No potential energy, 3 dimensional
space.
2
pi
2mi
pi2
1
Q
... exp(
)dp1...dp N dr1...drN
3N
N !h
i 2mi
1
N !h 3 N
3N
V
p
)dp dr1dr2 dr3
exp(
2mi
0
1 2mkT
N ! h 2
N
3N / 2
VN
We will get ideal gas law
pV= nRT
Semi-Classical Partition Function
The energy of a molecule is distributed in different modes
Vibration, Rotation (Internal : depends only on T)
Translation (External : depends on T and V)
Assumption 1 : Partition Function (thus energy distribution) can be separated
into two parts (internal + center of mass motion)
EiCM Eiint
EiCM
Eiint
Q exp(
) exp(
) exp(
)
kT
kT
kT
Q QCM ( N ,V , T )Qint ( N , T )
Semi-Classical Partition Function
Internal parts are density independent and most of the
components have the same value with ideal gases.
Qint ( N , , T ) Qint ( N ,0, T )
For solids and polymeric molecules, this assumption is not
valid any more.
Semi-Classical Partition Function
Assumption 2 : for T>50 K , classical approximation can be
used for translational motion
H CM
pix2 piy2 piz2
2m
i
U (r1 , r2 ,..., r3 N )
pix2 piy2 piz2
1
Q
... exp(
)dp 3 N ... (U / kT )dr 3 N
3N
N!h
2mkT
i
3 N
Z
N!
Configurational Integral
1/ 2
h2
2mkT
Z ... (U / kT )dr1dr2 ...dr3 N
Q
1
Qint 3 N Z
N!
Another, Different Treatment
Statistical Thermodynamics:
the basics
•
•
Nature is quantum-mechanical
Consequence:
–
–
•
•
Systems have discrete quantum states.
For finite “closed” systems, the number of
states is finite (but usually very large)
Hypothesis: In a closed system, every
state is equally likely to be observed.
Consequence: ALL of equilibrium
Statistical Mechanics and
Thermodynamics
Each individual
microstate is
equally probable
…, but there are not
many microstates that
give these extreme
results
Basic assumption
If the number of
particles is large (>10)
these functions are
sharply peaked
Does the basis assumption lead to something
that is consistent with classical
thermodynamics?
E1
E2 E E1
Systems 1 and 2 are weakly coupled
such that they can exchange energy.
What will be E1?
W E1 , E E1 W1 E1 W2 E E1
BA: each configuration is equally probable; but the number of
states that give an energy E1 is not know.
W E1 , E E1 W1 E1 W2 E E1
ln W E1 , E E1 ln W1 E1 ln W2 E E1
ln W E1 , E E1
0
E1
N1 ,V1
Energy is conserved!
dE1=-dE2
lnW1 E1
lnW2 E E1
E1
E1
N ,V
N
1
1
0
2 ,V2
ln W1 E1
ln W2 E E1
E1
E2
N1 ,V1
N2 ,V2
ln W E
E
N ,V
1 2
This can be seen as an
equilibrium condition
Entropy and number of
configurations
Conjecture:
S ln W
Almost right.
•Good features:
•Extensivity
•Third law of thermodynamics comes for free
•Bad feature:
•It assumes that entropy is dimensionless but (for
unfortunate, historical reasons, it is not…)
We have to live with the past, therefore
S kB ln W E
With kB= 1.380662 10-23 J/K
In thermodynamics, the absolute (Kelvin)
temperature scale was defined such that
1
S
E N ,V T
n
dE TdS-pdV idNi
i 1
But we found (defined):
ln W E
E
N ,V
And this gives the “statistical” definition of temperature:
ln W E
1
kB
T
E
N ,V
In short:
Entropy and temperature are both related to
the fact that we can COUNT states.
Basic assumption:
1. leads to an equilibrium condition: equal temperatures
2. leads to a maximum of entropy
3. leads to the third law of thermodynamics
Number of configurations
How large is W?
•For macroscopic systems, super-astronomically large.
•For instance, for a glass of water at room temperature:
W 10
210
25
•Macroscopic deviations from the second law of
thermodynamics are not forbidden, but they are
extremely unlikely.
Canonical ensemble
1/kBT
Consider a small system that can exchange heat with a big reservoir
Ei
ln W
ln W E Ei ln W E
Ei
E
E Ei
ln
W E Ei
WE
Hence, the probability to find Ei:
P Ei
W E Ei
exp Ei k BT
WE E
j
j
Ei
k BT
j
exp E j k BT
P Ei exp Ei kBT
Boltzmann distribution
Example: ideal gas
1
recall
Q N ,V , T Thermo
dr (3)exp U r
N!
N
N
3N
Helmholtz Free energy:N
1
V
dF3 N SdTdr p1dV 3 N
N!
N!
N
Free energy: Pressure
N
V
F
F ln 3 N P
VT N !
N
3
Energy:
N ln N ln N ln 3 N ln
Pressure:
V
F
F T
E
1 T Energy:
N
F
P
V T V
F 3N 3
E
Nk BT
2
Ensembles
•
•
•
•
Micro-canonical ensemble: E,V,N
Canonical ensemble: T,V,N
Constant pressure ensemble: T,P,N
Grand-canonical ensemble: T,V,μ
Each individual
microstate is
equally probable
…, but there are not
many microstates that
give these extreme
results
Basic assumption
If the number of
particles is large (>10)
these functions are
sharply peaked
Does the basis assumption lead to something
that is consistent with classical
thermodynamics?
E1
E2 E E1
Systems 1 and 2 are weakly coupled
such that they can exchange energy.
What will be E1?
W E1 , E E1 W1 E1 W2 E E1
BA: each configuration is equally probable; but the number of
states that give an energy E1 is not know.
W E1 , E E1 W1 E1 W2 E E1
ln W E1 , E E1 ln W1 E1 ln W2 E E1
ln W E1 , E E1
0
E1
N1 ,V1
Energy is conserved!
dE1=-dE2
lnW1 E1
lnW2 E E1
E1
E1
N ,V
N
1
1
0
2 ,V2
ln W1 E1
ln W2 E E1
E1
E2
N1 ,V1
N2 ,V2
ln W E
E
N ,V
1 2
This can be seen as an
equilibrium condition
Entropy and number of
configurations
Conjecture:
S ln W
Almost right.
•Good features:
•Extensivity
•Third law of thermodynamics comes for free
•Bad feature:
•It assumes that entropy is dimensionless but (for
unfortunate, historical reasons, it is not…)
We have to live with the past, therefore
S kB ln W E
With kB= 1.380662 10-23 J/K
In thermodynamics, the absolute (Kelvin)
temperature scale was defined such that
1
S
E N ,V T
n
dE TdS-pdV idNi
i 1
But we found (defined):
ln W E
E
N ,V
And this gives the “statistical” definition of temperature:
ln W E
1
kB
T
E
N ,V
In short:
Entropy and temperature are both related to
the fact that we can COUNT states.
Basic assumption:
1. leads to an equilibrium condition: equal temperatures
2. leads to a maximum of entropy
3. leads to the third law of thermodynamics
Number of configurations
How large is W?
•For macroscopic systems, super-astronomically large.
•For instance, for a glass of water at room temperature:
W 10
210
25
•Macroscopic deviations from the second law of
thermodynamics are not forbidden, but they are
extremely unlikely.
Canonical ensemble
1/kBT
Consider a small system that can exchange heat with a big reservoir
Ei
ln W
ln W E Ei ln W E
Ei
E
E Ei
ln
W E Ei
WE
Hence, the probability to find Ei:
P Ei
W E Ei
exp Ei k BT
WE E
j
j
Ei
k BT
j
exp E j k BT
P Ei exp Ei kBT
Boltzmann distribution
Example: ideal gas
1
recall
Q N ,V , T Thermo
dr (3)exp U r
N!
N
N
3N
Helmholtz Free energy:N
1
V
dF3 N SdTdr p1dV 3 N
N!
N!
N
Free energy: Pressure
N
V
F
F ln 3 N P
VT N !
N
3
Energy:
N ln N ln N ln 3 N ln
Pressure:
V
F
F T
E
1 T Energy:
N
F
P
V T V
F 3N 3
E
Nk BT
2
Ensembles
•
•
•
•
Micro-canonical ensemble: E,V,N
Canonical ensemble: T,V,N
Constant pressure ensemble: T,P,N
Grand-canonical ensemble: T,V,μ