* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Introduction (HL)
Homoaromaticity wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Kinetic resolution wikipedia , lookup
Aromaticity wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Hydroformylation wikipedia , lookup
Polythiophene wikipedia , lookup
Asymmetric induction wikipedia , lookup
Petasis reaction wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
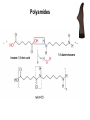
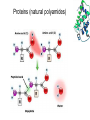
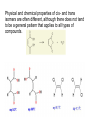
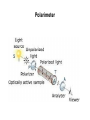
Introduction (HL) ● ● Amines are derivatives of ammonia, where 1, 2 or 3 hydrogens have been displaced by carbon chains. Amines are therefore classified as primary, secondary or tertiary. Nucleophilic substitution reactions ● ● ● http://www.youtube.com/watch?v=h5xvaP6bIZI http://www.youtube.com/watch?v=JmcVgE2W KBE Factors affecting the rate: ● a) ● b) c) Tertiary halogenoalkanes > secondary halogenoalkanes > primary halogenoalkanes halogenoalkane + water/OH- → alcohol dilute sodium hydroxide solution halogenoalkane + ammonia → amine halogenoalkane + cyanide → nitrile nitrile → amine nitrile → carboxylic acid Elimination reactions ● ● ● Removal of a small molecule from a larger molecule. A halogenoalkane reacts with a hot solution of NaOH dissolved in ethanol to produce an alkene. The hydroxide ion acts as a Bronstedt-Lowry base and accepts a proton. NOTE! Under different conditions halogenoalkanes react with OH - to form alcohols (substitution)! ● Therefore, altering the reaction conditions can give completely different products from the same reactants. Condensation reactions Two molecules react together to produce a larger molecule and a small by-product (often water). ● carboxylic acid + alcohol → ester ● Esters have distinctive smells (often fruity and sweet) and they are used in: ● Perfumes ● Artificial food flavourings ● Painkillers ● Solvents for paints carboxylic acid + amine → amide ● The bond is called a peptide bond and the product is called a dipeptide. Condensation polymerization ● The condensation can continue to form a polymer if each of the reacting monomer contains TWO functional groups that can undergo condensation. Polyesters ● Polyesters are made by condensing diols with dicarboxylic acids. Polyamides Demo: Synthesis of nylon ● Solution A: 0,5 g 1,6-diaminohexane 0,5 g sodiumcarbonate 25 ml water ● Solution B: 1 ml sebacoyl chloride 30 ml hexane ● Slowly pour the solution B into the solution A. Proteins (natural polyamides) Reaction pathways http://www.youtube.com/watch?v=41n3mDoVbvk Stereoisomerism ● ● ● Structural isomers have the same molecular formula but a different structural formula. Stereoisomers have the same structural formula but have the atoms arranged differently in space. There are two types of stereoisomerism: geometrical and optical isomerism. ● ● http://www.youtube.com/watch?v=RBtgAz70_J Y Geometrical isomerism ● ● Geometrical isomerism occurs with restricted rotation, i.e. when bonds are unable to rotate freely. Double bond: the pi-bond prevents rotation. Cycloalkanes: rotation is restricted due to the ring structure Physical and chemical properties of cis- and trans isomers are often different, although there does not tend to be a general pattern that applies to all types of compounds. Optical isomerism lemon orange ● ● ● Optical isomerism is shown by all compounds that contain at least one asymmetric carbon atom within the molecule. Chiral molecules are molecules that contain an asymmetric carbon atom. A carbon atom is asymmetric (=chiral) if it has four different atoms or groups bonded to it. Enantiomers ● The two mirror images, i.e. isomers, are called enantiomers. ● Enantiomers have similar chemical properties, unless they react with other optically active substances (as in the human body, where their physiological effects differ). ● Enantiomers differ in only one aspect of their physical properties: they rotate the plane of plane-polarized light in opposite directions: - laevorotatory enantiomers counterclockwise (to the left) - dextrorotatory clockwise (to the right) Polarimeter Racemic mixture ● ● ● If both enantiomers are equally present, it is called a racemic mixture or racemate. The two enantiomers rotate the plane of the polarized light by the same amount but in opposite directions. The rotations cancel each other out and the mixture appears to be optically inactive.