* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Attachment 2
Survey
Document related concepts
Transcript
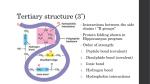
Teacher Outline Part I. The Hydrophobic Effect Key words to go over: electronegativity, covalent bonds, partial positive and negative charges, hydrogen bonds, hydrophobic effect, Second Law of Thermodynamics 1. Engage: Salad dressing experiment a. Shake oil and colored (balsamic/red wine) vinegar together and watch the two settle out into two layers. 2. Explain Critical Concept #1: Polarity affects the hydrophobicity or hydrophilicity of a molecule due to weak interactions called hydrogen bonds. a. Polar molecules are formed when atoms with different electronegativities, or attraction of electrons, are covalently bonded. This results in an uneven distribution of electrons across the molecule, creating partial positive and negative charges called dipoles. Electrons tend to localize around more electronegative atoms, creating a partial negative charge. i. Exploration and Application of Knowledge: Water contains two hydrogen atoms and one oxygen atom. The oxygen is very electronegative and therefore has a partial positive charge. b. This polarity affects the way the molecule interacts with other molecules, such as water. The hydrogen in water has a partial positive charge and interact weakly with other electronegative atoms (commonly oxygen and nitrogen), through a bond known as the hydrogen bond. Molecules that participate in hydrogen bonds are called hydrophilic, and those that do not are called hydrophobic. i. To remember: -philic = love, -phobic = fear. So hydrophilic molecules are water loving. c. Non-polar molecules are hydrophobic because they have equally distributed charge and thus cannot form hydrogen bonds. i. Evaluation/application of knowledge: Looking at the structure of vinegar and oil, which is polar and which is non-polar? Vinegar is polar and oil is nonpolar. 3. Explain Critical Concept #2: The Second Law of Thermodynamics states that a system in order will spontaneous return to disorder. This law guides the hydrophobic effect. a. The hydrophobic effect describes the tendency for hydrophobic molecules to aggregate together, away from hydrophilic molecules. b. Exploration and Application of Knowledge: Which state is more ordered in the salad dressing experiment? i. The system is at its most ordered state when it is fully shaken, since the vinegar is evenly distributed as droplets among the oil. Then the distinct layers form, the system is at a greater disorder. This may sound counterintuitive, but by creating that single interface between the oil and vinegar, more vinegar molecules are made available to the disordered system, forming hydrogen bond within themselves. When dispersed within the oil, there is a greater overall surface area that makes up the interface between the oil and vinegar, disrupting more hydrogen bonds between the vinegar molecules. Part 2. Bridging the gap by using surfactants Key words to go over: amphipathic molecules, surfactant, emulsion, surface tension 1. Engage: Salad dressing experiment with addition of detergent a. Shake with the addition of egg yolk (mustard or lecithin should work too) and compare to mixture without. 2. Explain Critical Concept #1: Amphipathic molecules are molecules that have both polar and non-polar properties. They can span across the interface of hydrophilic and hydrophobic layers, where the polar regions form hydrogen bonds with the hydrophilic layer. By supplementing hydrogen bonds at the hydrophilic interface, they reduce the surface tension thereby stabilizing the mixture. a. Exploration and Application of Knowledge: In an amphipathic molecule, which region is hydrophilic and which region is hydrophobic? The polar region is hydrophilic and the non-polar region is hydrophobic. Oil phase non-polar tail polar head Aqueous phase Amphipathic molecule b. The salad dressing mixture settles out until a surfactant (for example, egg yolk) is added, creating a type of solution called an emulsion, seen below in the diagram. 3. This lesson plan can be expanded to explain the ability of soap to clean grease! Part 3. Elaborate: Biological examples of the hydrophobic effect and amphipathic molecules in action! Key words to go over: hydrocarbons, macromolecules, lipids, phospholipids, membrane 1. Example #1: The cell membrane is composed of phospholipids, which are amphipathic molecules. a. A phospholipid is a type of lipid, which is a molecule that is largely composed of hydrocarbons. Hydrocarbons are just molecules that only contains carbon and hydrogen. Lipids are one of the four classes macromolecules that are the basis for cellular life. The other three are carbohydrates, nucleic acids, and proteins. b. Exploration and Application of Knowledge: What are some of the requirements of the cell membrane? Keeping organelles, nutrients, solutes inside to help maintain homeostasis of the cell. c. Exploration and Application of Knowledge: How may phospholipids arrange themselves to form the cell membrane (figure of a phospholipid structure is in handout)? i. They align side by side, forming a row and the two rows come together to form a lipid bilayer. The hydrophobic regions face inward and the polar regions face intracellular and extracellular water-filled fluid. 2. Example #2: Some types of nanoparticles are made by creating emulsions a. Recall the emulsion made in Part 2: The is the same way some nanoparticles are made in the lab! Instead of just shaking the mixture by hand, they add even more energy to the system by using two different machines, called homogenizers and sonicators. A homogenizer rapidly and vigorously blends the mixture and the sonicator uses sound energy to disturb the system. The high level of energy and the presence of surfactants allow the particles to get very small. 3. Example #3: Intro into drug delivery systems and pharmaceutics: Loading lipid-based nanoparticles with small molecule drugs allows for previously hydrophobic drugs to be given to patients intravenously. a. Engage: Insoluble molecule experiment i. Compare adding sugar and cotton balls to water. ii. Things that can form intermolecular interactions with water easily, are said to be soluble in water. iii. Disclaimer: This is a nuance to the ideas we learned in hydrophilicity segment, since the structure of cellulose shows that is is hydrophilic. 1. However, since cellulose exists in chains as a polymer, is forms an amorphous structure that wants to interact more with itself than water. b. Background: Many drugs are not very hydrophilic and thus insoluble in the blood, making them hard to give intravenously. Oral forms of drugs are difficult to make since the stomach is a very harsh, acidic environment that can break them down before they can be absorbed into the blood stream. Other issues include that they also can be very irritating to the gastrointestinal lining. Many cancer drugs and antibiotics need to be given intravenously. i. Exploration and Application of Knowledge: Many drugs that have been discovered are very hydrophobic. How may this be an issue when giving a drug intravenously? c. Lipids can assemble so that their hydrophobic tails aggregate around the hydrophobic drug, shielding it from the aqueous solution in the form of a particle. Recall: The Second Law of Thermodynamics is responsible for this assembly. These particles can be made very small, and are called nanoparticles. Nano- refers to nanometer. For reference, a human hair is about 7 micrometers, which is 1000x larger than 70 nanometers. There are two different basic types of lipid-based nanoparticles commonly made: micelles and liposomes. i. Engage: Watch this video from the NIH on nanotechnology and the relative scales of biology. https://www.nih.gov/research-training/nanotechnologynih. d. Exploration and Application of Knowledge: If you had a hydrophobic small molecule drug, where would you find them in the two nanoparticles in the figures below (handout has figures)? i. The hydrophobic drug would be found in the hydrophobic region of the nanoparticle, represented by the chains. These chains represent hydrocarbons that are very non-polar. Hydrophilic drugs can be either covalently bonded to the outer facing polar head groups of the liposome or micelle, or enveloped by the polar heads inside the liposome. (Optional) Part 4. Communicating with the public and other scientists. Create a class Instagram account where each group makes a series of posts to communicate what they learned during this class. Follow up each post with the following hashtags/tags: o #sciencecommunication –This allows students to see the other types of science people are communicating about on Instagram. o #instascienceclass – This hashtag currently has only 1 post listed on Instagram, and will allow students to see other classes in the area participating in this unique Insta-science classroom experience. o Tag my science outreach Instagram account in the description, @insta.sciencegrammar Encourage students to make posts such as: o Write a short summary of the most interesting thing that you’ve learned in class today. o Make a post about one of the explore or application questions. o Include images from the different experiments done throughout the lesson. o Propose other applications where you see these concepts at play in daily life. o Asking questions about the activities or content (that I will field and answer in the comments!)- make sure to tag @insta.sciencegrammar! A few weeks past the SciREN networking event, I will post a series of videos a nanoparticles being made, microscopic images of them after and their relevance in biomedical science! References: 1. Tymoczko, John L., Jeremy M. Berg, and Lubert Stryer. Biochemistry: a short course. Macmillan, 2011. 2. Whitten, K. W., Davis, R. E., Peck, M. L., and Stanley, G. G. Chemistry, 9 th edition. Cole Publishing, 2009. 3. Boundless. “Phospholipids.” Boundless Biology. Boundless, 30 Aug. 2016. Retrieved 09 Sep. 2016 from https://www.boundless.com/biology/textbooks/boundless-biologytextbook/biological-macromolecules-3/lipids-55/phospholipids-300-11433/. All other images found open source on Wikipedia or made by me.