* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Hypoplastic left heart syndrome with parchment left ventricle
Management of acute coronary syndrome wikipedia , lookup
Electrocardiography wikipedia , lookup
Heart failure wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Williams syndrome wikipedia , lookup
Coronary artery disease wikipedia , lookup
Echocardiography wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Down syndrome wikipedia , lookup
Aortic stenosis wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Myocardial infarction wikipedia , lookup
Marfan syndrome wikipedia , lookup
Turner syndrome wikipedia , lookup
Jatene procedure wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Congenital heart defect wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
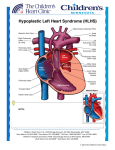
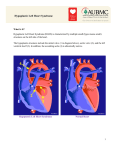
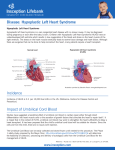
Journal of Medicine, Radiology, Pathology & Surgery (2015), 1, 26–28 CASE REPORT Hypoplastic left heart syndrome with parchment left ventricle: A rare perinatal autopsy case report K. R. Chatura1, G. V. Neethu1, K. S. Mavintop2 1 Department of Pathology, JJM Medical College, Davangere, Karnataka, India, 2Consultant Obstetrician, Mavintop Hospital, Davangere, Karnataka, India Keywords Autopsy, hypoplastic left heart syndrome, parchment left ventricle Correspondence Dr. K. R. Chatura, Department of Pathology, JJM Medical College, Davangere - 577 004, Karnataka, India. Email: [email protected] Received 18 January 2015; Accepted 25 February 2015 Abstract Hypoplastic left heart syndrome (HLHS) refers to the abnormal development of the left-sided cardiac structures, resulting in obstruction to blood flow from the left ventricular outflow tract. In addition, the syndrome includes underdevelopment of the left ventricle, aorta, and aortic arch, as well as mitral atresia or stenosis. The syndrome can be diagnosed by fetal echocardiography between 18 and 22 weeks of gestation. This is a fetal autopsy case report of HLHS, which was diagnosed on ultrasonography and medically terminated at 20 weeks of gestation. The autopsy and histopathological findings are discussed in detail. Some important aspects of epidemiology, prenatal diagnosis, and current literature on HLHS are highlighted. doi: 10.15713/ins.jmrps.12 Introduction Autopsy findings The most common major congenital anomalies are cardiovascular which occur in approximately 8 of 1000 births and account for approximately 20% of neonatal deaths and 50% of infant deaths, and are seen four to five times more frequently in stillbirths than in live-born babies.[1] Hypoplastic left heart syndrome (HLHS) is a rare uniformly fatal disease if it is not treated. No geographical or ethnic predilection in the worldwide distribution. Death results within few weeks after birth because of variable underdevelopment of the left ventricle that is unable to sustain the systemic circulation. In HLHS, the structural defect is variable but generally involves all or most components of the left side of the heart, i.e. the left atrium, mitral valve, and aorta are underdeveloped or absent.[2] Even in resource-limited countries, the use of echocardiogram has made it possible to make an accurate diagnosis of specific heart lesions, even though the multidisciplinary approach is still a mirage in these settings.[3] Parental consent for autopsy was obtained. The autopsy revealed the body to be that of a well-developed 430 g male infant of approximately 20 weeks’ gestation. External appearance was normal without any gross deformities. The baby was cyanotic. Abnormal fluid collection in pleural and peritoneal cavities was seen. All body organs were present and in normal anatomical position; there was no malrotation of the organs. The most notable findings involved the heart, which was abnormally shaped, weighed 10 g with measurement of 2 cm × 1 cm × 1 cm, the left ventricle was pale and markedly thinned out [Figure 1]. Right ventricle was thickened with both aorta and pulmonary artery arising from it. Aorta was equal in size with pulmonary artery suggesting aortic atresia. Both ventricular and atrial septal defect and patent ductus arteriosus were present. Left atrium was hypoplastic with mitral atresia. Histological examination showed absence of myocardium in the free left ventricular wall with epicardium separated by only wisps of cardiac muscle from the endocardium [Figure 2]. Case Report We report a case of fetal autopsy with left hypoplastic heart syndrome, which was antenatally confirmed on ultrasonography (USG) and hence medically terminated at 20 weeks of gestation in a 23-year-old mother who was gravida 3, para 0, and abortion 3. 26 Discussion Lev in 1952, termed HLHS initially as hypoplasia of the aortic tract complex. HLHS was first used in 1958, by Noonan and Nadas to collectively describe their series of specimens with Journal of Medicine, Radiology, Pathology & Surgery ● Vol. 1:2 ● Mar-Apr 2015 Chatura, et al. Figure 1: Markedly thinned out left ventricle Figure 2: Absence of myocardium in free left ventricular wall with epicardium separated by only wisps of cardiac muscle from the endocardium (H and E, ×40) multiple malformations involving left-sided structures of the heart. HLHS refers to the abnormal development of the left-sided cardiac structures, resulting in obstruction to blood flow from the left ventricular outflow tract. The syndrome also includes underdevelopment of the left ventricle, aorta, aortic arch, mitral atresia or stenosis. As in most congenital heart diseases, the embryologic cause is not fully known. A multifactorial influence is attributed to be the cause of up to 90% of cardiac anomalies, with a 2% to 6% recurrence rate in further offspring.[4] A normal fetus has a parallel circulation that adequately supports single-ventricle physiology before birth. Three communications (the ductus venosus, foramen ovale, and ductus arteriosus) shunt oxygenated placental blood largely Journal of Medicine, Radiology, Pathology & Surgery ● Vol. 1:2 ● Mar-Apr 2015 Hypoplastic left heart syndrome past the hepatic and pulmonary beds to supply the splanchnic circulation. HLHS is well supported in this situation, and as a result, it is rarely a cause of fetal demise. And hence HLHS is probably a secondary result of early obstructive lesions of either mitral or aortic valvular development. However, the primary cause of the obstructive flow lesion that leads secondarily to HLHS is unknown. This argues for a complex early multifactorial event or, more likely, a transient early insult.[5] Patients are categorized into three primary subsets on the basis of atrioventricular and semilunar valvular morphology: 1. Aortic atresia with mitral atresia (40%), 2. Aortic stenosis with mitral stenosis (30%), and 3. Aortic atresia with mitral stenosis (30%)[5] The said fetus had both aortic atresia and mitral atresia and was supported by ductus venosus, foramen ovale, and ductus arteriosus. The free left ventricular wall was parchment like, an unusual feature, often described in the right ventricle in cases of Uhl’s syndrome.[6] Histological examination confirmed the absence of muscle, a thin layer of elastic and fibrous tissue being all that separated the epicardium, and the endocardium. Prenatal echocardiography can be used to detect unbalanced ventricles as early as 20 weeks of gestational age. Postnatal echocardiography establishes the diagnosis and guides medical and surgical decision-making.[5] An increase in the number of cases in which HLHS is detected prenatally is seen because of the widespread use of ultrasound screening for fetal malformations. Despite this advantage which provides the opportunity to plan perinatal management, the outlook for these fetuses is still poor, with an overall survival rate below 40% and highlights the importance of presenting these figures when counseling parents with affected foetuses. Several factors explain the discrepancies between fetal and surgical series. These limitations worsen with the negative feedback generated by the high rate of termination of pregnancy (TOP) following prenatal diagnosis of HLHS.[7] With the incidence of HLHS about 0.1-0.25/1000 live births, in the Indian scenario, we should expect about 2000 babies with HLHS born every year. In reality, the total number of babies with HLHS seen in major centers in India will not be more than 100 children/year. Most of the fetuses with a diagnosis of HLHS get terminated by parents’ options and counseling offered to them by perinatologists.[8] The main reason for referral was suspected heart defect on a routine ultrasound scan (82%). The mean gestational age at diagnosis was 21 weeks. Most cases were detected at ≤22 weeks (72%), the upper limit for TOP in Spain. 79% (58/73) of the cases in which HLHS was detected at ≤22 weeks were terminated.[7] HLHS constitutes 5% of all cases of congenital heart disease and is responsible for 25% of cardiac deaths in the 1st week of life. Among 10,000 live births, approximately 1.8 will be born with HLHS, with a slight male predominance. The recurrence risk is 2.2% for one affected sibling and 6% for two affected siblings, suggesting a genetic predisposition.[5] Autosomal recessive, autosomal dominant, and polygenic inheritances have been suggested. Associated extracardiac 27 Hypoplastic left heart syndrome abnormalities of craniofacial, gastrointestinal, genitourinary, and central nervous system are seen. Prenatal diagnosis with good counseling is necessary because of high perinatal mortality. A karyotype analysis and detailed ultrasonographic scan should be done to rule out other anomalies.[9] In our case, prenatal USG established the diagnosis and guided the decision of termination. Autopsy examination demonstrated the HLHS without extracardiac anomalies. In view of the previous pregnancy loss, and associated recurrence risk counseling and karyotyping was advised. References 1. Ananadumar C, Nuruddin M, Wong YC, Chia D. Routine screening with fetal echocardiography for prenatal diagnosis of congenital heart disease. Ultrasound Rev Obstet Gynecol 2002;2:50-5. 2. Barron DJ, Kilby MD, Davies B, Wright JG, Jones TJ, Brawn WJ. Hypoplastic left heart syndrome. Lancet 2009;374:551-64. 3. Bugaje MA, Danbauchi SS. Hypoplastic left heart syndrome: A case report and review of the literature. Niger J Basic Clin Sci 2012;9;33-5. 28 Chatura, et al. 4. Connor JA, Thiagarajan R. Hypoplastic left heart syndrome. Orphanet J Rare Dis 2007;2:23. 5. Gruber PJ, Spray TL. Hypoplastic left heart Syndrome. In: Yuh DD, Vricella LA, Baumgartner WA, editors. The John Hopkins Manual of Cardiothoracic Surgery. New York: The McGraw-Hill Companies Inc.; 2007. p. 1231-40. 6. Uhl HS. A previously undescribed congenital malformation of the heart: Almost total absence of the myocardium of the right ventricle. Bull Johns Hopkins Hosp 1952;91:197-209. 7. Galindo A, Nieto O, Villagrá S, Grañeras A, Herraiz I, Mendoza A. Hypoplastic left heart syndrome diagnosed in fetal life: associated findings, pregnancy outcome and results of palliative surgery. Ultrasound Obstet Gynecol 2009;33:560-6. 8. Kulkarni S, Rao S. Definitive therapy for hypoplastic left heart syndrome - Indian scenario. Indian Heart J 2012;64:338-40. 9. Sefa K, Bulent Y. Hypoplastic left heart syndrome. J Obstet Gynecol India 2006;6:76-8. How to cite this article: Chatura KR, Neethu GV, Mavintop KS. Hypoplastic left heart syndrome with parchment left ventricle: A rare perinatal autopsy case report. J Med Radiol Pathol Surg 2015;1:26-28. Journal of Medicine, Radiology, Pathology & Surgery ● Vol. 1:2 ● Mar-Apr 2015