* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 17 lecture notes on Chemical Equilibria
Electrolysis of water wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Supramolecular catalysis wikipedia , lookup
Asymmetric induction wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Electrochemistry wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Process chemistry wikipedia , lookup
Marcus theory wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Vapor–liquid equilibrium wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Thermomechanical analysis wikipedia , lookup
Chemical reaction wikipedia , lookup
George S. Hammond wikipedia , lookup
Thermodynamic equilibrium wikipedia , lookup
Click chemistry wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Thermodynamics wikipedia , lookup
Rate equation wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Stoichiometry wikipedia , lookup
Transition state theory wikipedia , lookup
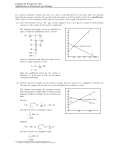
17 Chemical Equilibria Consider the following reaction: aA + bB ---> cC + dD As written is suggests that reactants A + B will be used up in forming products C + D. However, what we learned in the section on thermodynamics is that a reaction might not occur to completion to leave only C + D. Instead it can form any proportion of A and B to C and D, including remaining primarily as the products. 17-1 Consider a system in which every compound is present: A better way of considering the reaction is to recognize that from a starting point with A and B placed in a closed thermodynamic system, a reaction proceeds to the point in time at which four compounds, A, B, C and D come to exist simultaneously. This is shown in the next graph. 17-2 17-3 Extracting kinetics from the plot: This plot provides us with enormous information about both the kinetics and the thermodynamics of the reaction. From a kinetic perspectives we know that the reaction proceeds at a rate determined by - 1 d[A] rate = = a dt 1 d[C] c dt It changes rapidly at first and then levels off later in time. 17-4 Kinetics when nothing seems to be happening: What is of greater interest in this chapter is the rate of reaction at the point where there appears to be no change in concentration of reactants and products. In fact, at that point, we can consider two reactions to be occurring, a forward reaction aA + bB ----> c C + dD and a reverse reaction cC + dD ----> aA + bB 17-5 Deriving the Equilibrium Constant, Keq, from Rate Constant, k. To simplify things, consider reactions following simple one step bimolecular mechanisms. Then we have rate laws of the following form: Rate = kf[A][B] forward reaction Rate = kr[C][D] reverse reaction and 17-6 A little bit of math and presto: Keq Obviously from the plot, at the point where all of the curves flatten out, the rates at which A, B, C and D are formed are the same. Thus we can equate the two rate laws kf[A][B] = kr [C][D] and rearranging Kc = kf __ kr = 17-7 [C][D] ______ [A][B] The constant we will use the rest of the semester. The ratio, Kc, is a very important value know as the constant. equilibrium How important is it? Coming to understand and apply this constant will be the primary focus of THE REST OF THE COURSE. We will come to learn that though Kc is derived from the kinetics of the reaction, it is an important thermodynamic constant which is directly related to the most important of thermodynamic state functions, the Gibbs free energy, ∆Go. 17-8 Generalizing the equilibrium constant, Kc. It can be shown that no matter what the mechanistic pathway of a reaction, the equilibrium constant, Kc, is defined as aA + bB ⇔ cC + dD Kc [C]c[D]d = [A]a[B]b for any reactants and products AT EQUILIBRIUM. 17-9 Example. reaction: What is the equilibrium expression for the following H2(g) + I2 (g) ⇔ 2HI (g) [HI]2 Kc = [H2][I2] 17-10 A new way to describe all chemical reactions, the equilibrium expression: Note that every reaction ultimately arrives at an equilibrium; i.e., the point at which the forward and reverse reactions have equal rates so that there is no change in the overall concentration of species present in the system. Thus every reaction has an equilibrium constant, Kc. A few hundred equilibrium constants for the work we will do in Chapters 18-20 are found in Appendixes F, H and I of Davis. 17-11 The magnitude of Keq values Equilibrium constants can vary enormously in magnitude. Very large and very small numbers are possible: Cu++ + S= HPO4-2 ⇔ CuS ⇔ H+ + PO4-3 Kc = 1.1 x 1035 Kc = 3.5 x 10-13 Note that we can rewrite an equilibrium expression as the reverse reaction and the new Kc’ is calculated simply as the reciprocal of Kc. 17-12 Example. The equilibrium expression for the formation of CuS is written below: [CuS] ++ = 35 Cu + S ⇔ CuS(s) Kf = 1.1 x 10 = [Cu++] [S=] From this information, determine the equilibrium constant for the dissociation of CuS. The dissociation of CuS is written as [Cu++] [S=] CuS(s) ⇔ Cu++ + S= Kd = = 1/Kf = 9 x 10-36 [CuS] 17-13 A few points to make about Kc: 1. Temperature dependence. The value is obtained for a specific temperature. This is not surprising since K is a thermodynamic constant and all thermodynamic constants are strongly dependent upon T. Once again, a standard temperature has to be selected for recording values in the Appendix. The typical temperature used is 25oC. Later we will learn to use the van’t Hoff equation to convert between Kc values at different temperatures. 17-14 2. In performing equilibrium calculations: i. Pure solids and pure liquids are assigned an activity of 1 ii. For ideal solutions, the activity of samples dissolved in solution is given in molarity iii. For ideal gases, the activity of samples is given as partial pressures in atm. 17-15 3. Equilibrium constants are dimensionless 17-16 . 4. Looking at K to determine reaction spontaneity. If the value for K>1, the formation of products is favored over reactants. If the value for K<1, the formation of reactants over products is favored. 17-17 Example. Calculation of equilibrium constant. One liter of an equilibrium mixture is found to contain 0.172 moles of PCl 3, 0.086 moles of chlorine and 0.028 moles of PCl5. Calculate Kc for the reaction. PCl5 ⇔ PCl3 + Cl2 [PCl3][Cl2] (0.172mole/1l)(0.086mole/1l) Kc = = = 0.53 [PCl5] (0.028mole/1l) 17-18 Example. Calculation of equilibrium constant. The decomposition of PCl5 at a different temperature involves introduction of one mole of PCl 5 to an evacuated 1 liter container. At equilibrium, 0.6 moles of PCl3 is present in the container. Calculate Kc . PCl5 ⇔ PCl3 + Cl2 initial 1M 0M 0M change -0.6M +.6M +.6M equilibrium 0.4M 0.6M 0.6M (0.6M)(0.6M) Kc = = 0.9 (0.4M) 17-19 Example. Calculation of equilibrium constant. In an evacuated 1 liter container, 0.8 moles of N2 and 0.9 moles of H2 are placed. At equilibrium, 0.2 mole of NH3 is present. Calculate Kc. N2 + 3H2 ⇔ 2NH3 initial 0.8M 0.9M 0 change -0.1M -0.3M +0.2M equilibrium 0.7M 0.6M 0.2M (0.2M)2 Kc = = 0.26 (0.7M)(0.6M)3 17-20 Relating the Reaction Quotient, Q, to Kc. The calculation of Kc is accomplished by ratioing the equilibrium concentration of products to reactant. However, nothing stops us from making a calculation of the ratio of products to reactants at any non-equilibrium position along the reaction path. The ratio of products to reactants is generally called the mass action equation, Q, or in Davis, the reaction quotient. Q [C]c[D]d = [A]a[B]b Recall that at equilibrium, Q ------> K 17-21 Some rules for relating Q to K We can calculate the value of Q anywhere along the course of the reaction. Note that in the reaction of A and B forming C and D, the value of Q starts off very small (reactants in denominator) compared to the final K value. Q increases until the point that the forward and reverse reaction rates are equal, and the system is at equilibrium; then Kc can be calculated. We can establish the following rules for describing where the reaction is relative to equilibrium by comparing the value of Q to K. Q<K Q = K Q >K forward reaction dominates (example above) system at equilibrium reverse reaction dominates 17-22 17-23 Example. Calculating Q. The equilibrium constant for the following reaction, Kc, is 49 at 450oC. If 0.22 mole of H2, 0.22 mole of I2 and 0.666 mole of HI were put into an evacuated container, would the system be at equilibrium? If not, in what direction must the reaction proceed to reach equilibrium? H2 + I2 ⇔ 2HI (assume one liter) [HI]2 (0.666M)2 Q = = = 9.2 [H2][I2] (0.22M)(0.22M) Q<K ∴ rxn proceeds to right to form more [HI] 17-24 Example. The equilibrium constant, Kc, is 71.00 for the reaction below. If 0.6 mole of SO2 and 0.2 mole NO2 are placed into an evacuated 2.0 liter container and allowed to reach equilibrium, what will be the concentration of each compound at equilibrium? ⇔ SO3 + NO SO2 + NO2 Initial 0.3M 0.1M 0M 0M Change -xM -xM +xM +xM xM xM Equilibrium (0.3-x)M (0.1-x)M x2 x2 Kc = 71 = = (0.3-x)(0.1-x) 0.03-.4x+x2 0 = 70x2-28.4x+2.13 solve quadratic to get x = .0993 [NO2]=0.0007M [SO2] = 0.2M [NO] = [SO3] = 0.1M 17-25 Example. The equilibrium constant is 49 for the reaction below. If 1.00 mole of HI is placed into a 1.0 liter container and allowed to reach equilibrium, what will be the equilibrium concentration of each compound? H2 + I2 ⇔ 2HI Initial 0M 0M 1M Change +xM +xM -2xM Equilibrium xM xM (1-2x)M (1-2x)2 Kc = = 49 x2 use quadratic equation [H2] = 0.11M x = 0.11 [I2] = 0.11M [HI] = 0.78M 17-26 Stress and LeChatlier’s Principle: Stress is a big deal, in real life and in chemical equilibria. What do we do in life when things change? We do what we can to relieve the stress by moving away from it. We apply the same notion to chemical equilibria. In a system at equilibrium, when a change occurs a stress is imposed on the system. The system then responds by adopting new equilibrium conditions that relieve the stress. This is the definition of LeChatlier’s Principle: If a change in conditions occurs to a system at equilibrium, the system responds to relieve the stress and reach a new state of equilibrium. What are some of the ways stress is introduced to a system? 1. Changes in concentration of system components. 2. Change in system temperature. 3. Change in volume or pressure (in gaseous systems.) 17-27 Case 1. LeChatlier’s Principle: Changes in Concentration Consider the general reaction: A + B ⇔ C + D Suppose we add additional reactant A or B to the system. This means we have more reactant than is necessary to establish equilibrium and the system shifts to reduce the amount of A and B. This results in an increase in the amount of C and D. The counter to this is also true. If the system changes by adding C and D to the system, the equilibrium shifts to the left forming more A and B. Looking at the equilibrium constant equation gives us a mathematical justification for the direction of the change. [C] [D] ___________ Kc = [A] [B] Note for example that if we increase the amount of A, then to maintain the equality, the equilibrium must shift so that the amount of C and D go up as well. What happens to the amount of B? It must go down. 17-28 Some General Rules for LeChatelier and Concentration Changes. From a chemical perspective, we have an equilibrium system with relative concentrations of compounds defined by Kc. We impose a stress on the system by changing a concentration and create a nonequilibrium Q value for the system. However, because of the nonzero free energy now in the system, Q will in time return to K as ∆G ---> 0. We can make a little table telling us how equilibrium adjust to compensate for changes in concentration. A + B ⇔ C case 1: Add A B decreases C increases case 2: A decreases Add B C increases case 3: A increases B increases Add C case 4: A increases B increases C decreases 17-29 concentrations + D D increases D increases D decreases Add D Example: LeChatlier’s Principle applied to a change in concentration. A 2 liter vessel contains an equilibrium mixture of 1.2 mole of COCl2, 0.6 mole of CO and 0.2 mole of Cl2. First calculate the equilibrium constant for the reaction: CO initial equilibrium: 0.3M + Cl2 ⇔ 0.1M [COCl2] Kc = = 20 [CO] [Cl2] 17-30 COCl2 0.6M An additional 0.8 mole of Cl2 is added to the vessel. Calculate the molar concentration of CO, Cl2 and COCl2 when the new equilibrium is established. CO + Cl2 ⇔ COCl2 Original equilibrium 0.3M 0.1M 0.6M Stress added 0M +0.4M 0M New initial conc. 0.3M 0.5M 0.6M Change (0.3-x)M (0.5-x)M (0.6+x)M New equilibrium 0.121M 0.321M (0.6) 1) After stress Q = = 4 (0.3)(0.5) Q<K so rxn proceeds forward (0.6+x)(0.6+x) 2) K = 20 = = (0.3-x)(0.5-x) 0.15-0.8x+x2 solve quadratic, 0 = 20x2 - 17x + 2.4, to get x = 0.179 17-31 0.779M Case 2. LeChatelier’s Principle: Changes in Temperature. Consider the following exothermic reaction. A + B ⇔ C + D + heat (∆H is -) This means that heat is being generated by the reaction. If we add additional heat to the system, the stress of the increased temperature will make the system respond to try and to cool things down. This means the system reaction will shift to the left to reduce the heat generated by the exothermic process. 17-32 We can use similar sorts of reasoning to establish the following rules: exothermic rxn exothermic rxn endothermic rxn endothermic rxn temperature increases temperature decreases temperature increases temperature decreases 17-33 reaction shifts left reaction shifts right reaction shifts right reaction shifts left Examples: How will an increase in temperature affect each of the following reactions? 2NO2(g) ⇔ N2O4 + heat Exothermic reaction so equilibrium shifts to the left to reduce the temp. of the system. H2 (g) + Cl2 (g) ⇔ 2HCl (g) +92 kJ Exothermic reaction so equilibrium shifts to the left to reduce the temp. of the system. H2 (g) + I2 (g) ⇔ 2HI ∆H = +25kJ Endothermic reaction so equilibrium shifts to the right to reduce the temp. of the system. 17-34 Case 3. LeChatlier’s Principle: Changes in Volume and Pressure (application to reactions involving gases) First note from the ideal gas law that P and V are inversely related. If we reduce the volume of a system, this has the affect of increasing the concentration of a gas in the system. The system will thus respond by shifting the reaction in the direction to reduce the concentration of gases in the system. This means the reaction shifts toward the side that has the fewest moles of gas at equilibrium Similarly reasoning yields the following: V decreases (P, [ ] increase) V increases (P, [ ] decrease) shift reaction toward side with smaller number of moles of gas shift reaction toward side with larger number of moles of gas Note that if we assume an ideal gas, then if there are an equal number of moles of gas on either side of the reaction, then pressure or volume changes have no affect on the equilibrium. 17-35 Examples. How will an increase in pressure (concentration) resulting from a decrease in volume affect the following equilibria? H2 (g) + I2 (g) ⇔ 2HI (g) ∆n = 2-2 = 0 therefore, no change in equilibrium 4NH3 (g) + 5O2 (g) ⇔ 4NO (g) + 6H2O (g) ∆n = 10-9 = 1, therefore, shifts to the left to reduce moles of gas. PCl3 (g) + Cl2 (g) ⇔ PCl5 (g) ∆n = 1-2 = -1, therefore, shifts to the right to reduce moles of gas 2H2 (g) + O2 (g) ⇔ 2H2O (g) ∆n = -1 therefore, shifts to the right to reduce moles of gas 17-36 Equilibrium Constant, Kp, Expressed for Gases: Taking a look at the ideal gas law: PV = nRT The equation suggests a way to relate partial pressure, P, of a gas in the system to concentration, [ ] by [ ] = n/V = P/RT Substituting this relationship into the general equilibrium equation yields: aA (g) + bB(g) ⇔ cC (g) + dD (g) (PC)c (PD)d Kp = ____________________ (PA)a (PB)b The relationship between Kc and Kp follows from the ideal gas law: Kp = Kc(RT)∆n ∆n =(ngas prod - ngas react) where 17-37 Example: For the reaction below with a Kc = 20 at 25oC, what is Kp? CO + Cl2 ⇔ COCl2 ∆n = 1-2 = -1 Kp = Kc(RT)∆n = 20[(0.082atml/molK)(298K)]-1 = 0.82 17-38 Example: A calculation interchanging Kc and Kp Kc is 49 for the following reaction. If 1.0 mole H2 and 1 mole I2 are allowed to reach equilibrium in a 3 liter vessel, how many moles of I2 are unreacted at equilibrium? H2 (g) + I2 (g) ⇔ 2HI (g) initial 0.33M 0.33M change 0.33-xM 0.33-xM +2xM equilibrium 0.073M 0.073M 0.514M [HI]2 4x2 Kc = 49 = = [H2][I2] 0.01089-0.66x+x2 0M 45x2-32.34x+5.34 = 0 solve quadratic x = 0.257 3(0.257) = 0.219 moles of reacted I2 17-39 Now what are the equilibrium pressures of H2, I2 and HI? ∆n = 0, therefore, Kc = Kp [HI]2 ______ [I2][H2] PHI2 = ______ PI2 PH2 0.264(PI22) = PHI2(0.0053) So moles I2 = moles of H2, therefore, (2.2(PI2) )2 49 = PI22 PI2 = 0.044 = PH2 And 2.2(PI2) = PHI so PHI = 0.0968 17-40 Relating Kc to Thermodynamic Quantities through Go: Recall that in the last chapter we introduced the idea of the free energy of the system, ∆Go, under standard conditions. ∆Go describes the free energy change in the system that accompanies the conversion of all reactants in their standard states (1M, 1 atm) to products in their standard states (1M, 1 atm). We now define the free energy, ∆G, which is the free energy of a reaction at concentrations other than 1M or 1 atm. It is found from ∆Go by correcting for non-standard conditions using the reaction quotient, Q: ∆G = ∆Go + RT ln Q 17-41 The special case of thermodynamics at equilibrium. Remember that at equilibrium, ∆G = 0 and Q = K. This means the above equation reduces to: ∆Go = -RT ln K (at equilibrium) Among other things this relationship tells us that we can calculate the equilibrium constant, Kc, from the thermodynamic constants in Appendix K. 17-42 Examples: Converting between Kc and Go Case 1. ∆G of = 2C graphite + 3H2 (g) + O2 (g) D C2H5OH 1 2 -168.6 Kc = exp ( Kc = exp ∆G kKJ o RT mol ) −168.6x103 ( ) (−8.3)(298K ) Kc = 3.5 x 1029 So a spontaneous reaction has a K > 1 17-43 Case 2. ∆G of N2(g) D 2N(g) = 456 kKJ mol Kc = exp ( −456x103 ) (8.3)(298) Kc = 8.6 x 10 –81 So a non-spontaneous reaction has K < 1. Reaction is spontaneous in opposite direction. 17-44 What generalization can be made about ∆Go and Kc? If ∆Go < 0 then K>1 If ∆Go > 0 then K< 1 products favored over reactant reactants favored over products Isn’t it exciting to see that chemical equilibrium is another model for describing thermodynamics? In this case we don’t measure the change in energy, instead we measure the equilibrium concentrations. 17-45 End-of chapter equations and the Famouns Scientists who invented them: It is the end of the chapter and it is time to throw a hard equation at you. Just to let you know that this is a pretty common occurrence, consider: end of chapter equation name ClausiusClapeyron Arrhenius van’t Hoff if we then for the we get a new know this: following change: physical constant ∆Hvap ∆T P(vapor pressure) ∆T ∆T Ea ∆H 17-46 rate constant equilibrium constant The van’t Hoff Equation: Finding a new Kc at a new T: Of interest in Chapter 17 is the bottom equation: the van’t Hoff equation that allows us to determine Kc for a chemical system at any temperature we desire. KT2 ∆Ho 1 1 ln = . - KT1 R T1 T2 Notice that this is the quantitative way to look at LeChatelier’s principle applied to changes in temperature. 17-47 Example: Using the van’t Hoff equation to find a new K. For the reaction below, 2NO2 ⇔ 2NO + O2 ∆Ho = 114 kJ/mole and Kp = 4.3 x 10-13 at 25oC. So what is Kp at 250oC? Insert T2 = 523K, R = 8.312 J/mol K. ∆Ho = 114 kJ/mole and Kp = 4.3 x 10-13 at 25oC. KT2 = KT1 [exp ( ∆H ( 1 − 1 ))] R T1 T2 exp (1n ( KT )) = exp [ ∆H R 2 KT1 -13 KT2 = (4.3 x 10 ) exp So at 250oC, (1 T1 114x103 ( 8.314 ( 1 ) T2 ] 1 1 − )) 298 523 KT2 = 1.7 x 10-4 Is this consistent with what you know from LeChatelier? 17-48