* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Infectious Bursal Disease of Chickens
Survey
Document related concepts
Rotaviral gastroenteritis wikipedia , lookup
Swine influenza wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Hepatitis C wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
2015–16 Zika virus epidemic wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Orthohantavirus wikipedia , lookup
Influenza A virus wikipedia , lookup
Ebola virus disease wikipedia , lookup
Antiviral drug wikipedia , lookup
West Nile fever wikipedia , lookup
Hepatitis B wikipedia , lookup
Marburg virus disease wikipedia , lookup
Herpes simplex virus wikipedia , lookup
Transcript
Infectious Bursal Disease of Chickens
By KA TSUYA HIRAI
Faculty of Agriculture, Gifu University
Infectious bursa] disease (IBD), formerly
termed Gumboro disease had been observed
in the U.S.A. towards the end of the 1950
but it was not until 1962 that it was first
described by Cosgrove. At first the disease
was erroneously called "Avian nephrosis". The
disease is a viral disease of young chickens
for which the causative virus is tentatively
classified as a member of Reoviridae. The
virus induces atrophy of the bursa of Fabricius (BF) as a result of necrosis of lymphocytes and also causes a general lymphocidal effect in other lymphoid organs, including the thymus and spleen. The BF has a
key role in the development and maturation
of the humoral immune response of the
chicken . A number of workers have demonstrated that infection with IBD virus at a
young age can lead to immunosuppression.
Therefore, this disease is of interest immunologically. In the present paper some of our
recent studies will be described briefly.
Morphological characterization
of the virion2 •4 •7 >
The virus was tentatively classified as a
member of Reoviridae. The classification was
based upon the morphology under ultrathinsection electron microscopy of the BF from
infected chickens and upon limited biochemical characterization. Litte imformation is
available on the ultrastructure of the IBD
virus. In this section, electron micrographs
of purified IBD virus, negatively stained with
phosphotungstate, are presented.
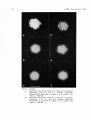
IBD virus apparently has an outer layer,
as indicated by the large size of intact particles with hexagonal outlines (Plate 1 a-f).
Moreover, the capsomeric detail on the main
capsid surface of I BD virus often appeared
partially obscured by such a layer. This outer
layer was very thin (7 to 8 nm) and continuous, with T-shaped structures suggestive
of the configuration reported for rotavirus
but not as clearly defined1 2,1v.1S> Although the
outer layers of reovirus and orbivirus are also
indistinct, they are featureless11.1 s> . The outer
layer of IBD virus may exert some stability
to the structure of the underlying major capsid layer. By rotational enhancement of image
detail (Plate 2 a-f) , the results of this study
confirmed our previous report that IBD virus
has an icosahedral symmetry of T=3, with a
probable 32 large capsomeres. This type of
symmetry of the unique feature of subunit
sharing are morphological characteristics reported for the Reoviridae famiJyH,10>. The
results obtained are summarized as follows.
An outer layer surrounding the capsid of IBD
virus was evident from electron micrographs
of intact virus particles having diameters of
62 to 63 nm. The capsid was found to be
composed of large morphological units or capsomeres, measuring about 12 nm in diameter.
The architecture of the capsid appears to be
that of T=3 symmetry, with probable 32
morphological units by rotational enhancement of image detail.
Structural proteins of the virion3•1 >
Apart from the initial study by Nick et
aJ.13>, little information is available on the
structural proteins of IBD virus, using polypeptide gel electrophoresis. The exact number of polypeptides and their locations within
the virion are not yet clearly understood. The
results of this study demonstrated that
47
Plate 1. Electron micrographs of negatively stained IBD virus particles.
a. A large number of IBD virus recovered from the 1. 34 g/ ml
CsCl fraction. Bar = 100 nm. X 45,000.
b. Intact virions of IBD virus showing hexagonal outlines.
Bar = 100 nm. X 130,000.
c. Intact virions of lBD virus with an outer layer. Bar= 50nm.
X330, 000.
d. Higher magnification of an intact virion showing the outer
layer. The surface of the particles appear to have T - shaped
morphology which is continuous. Bar= 50 nm. X 450, 000.
e. IBO virus particles recovered from the 1. 35 g/ ml CsCl
fraction. Intact virions of IBO virus (arrow) and particles
with clear capsomeres showing the Joss of the outer layer
(double arrow). Bar= 100 nm. X 190,000.
f. Particles showing clearly discernible capsomeres.
Bar = 100 nm. X 200,000.
48
JARQ
Vol. 14, No. 1, 1980
Plate 2. continued
a- c. Single IBO virus particle viewed on a 3- hold axis of symmetry
representing n = O, n = 6, and n = 5 rotations, respectively.
Enhancement of capsomeres is evident in n = 6 rotation. Bar
= 50 nm. X390, 000.
d- f. Single IBO virus particle viewed on a 5- fold axis of symmetry
representing n = O, n = 5, and n = 6 rotations, respectively
Enhancement of capsomeres is evident in n = 5 rotation. Bar
= 50 nm. X390, 000.
49
Table 1. A comparison of molecular weights*
of IBD virus proteins with those of
rotavirus, bluetongue virus and avian
reovirus
IBDV
Human
rotavirus
Bluetongue
virus
Avian
reovirus
127
103
97
88
58
32
26
21
140
110
101
82
61
42
29
140
125
115
85
133
124
98
51
33
26.5
23
* Molecular
72
40
36
32
weight Xl03
structural proteins of IBD virus consist of
seven species, two major and five minor polypeptides. These are Pl to P7, with molecular
weights of 133X103, 124X l 0 3, 98Xl03, 51X
103, 33X10:!, 26Xl03 , and 23Xl03, respectively. The major polypeptides were associated
with the smaller subunit particles and stringlike structures. Therefore, these polypeptides
may be the capsomeral proteins of IBD virus.
These results differed from t hose reported by
Nick et aJ.1 3> with regard to the number of
polypeptides and their molecular weights.
Only fou r polypeptides were found in the
previous study, three of which did compare
closely in molecular weight with our P4, P5,
and P6 proteins. However, a polypeptide having a molocular weight of 11 x 103 was not
detected in ou1· preparations. The limited
amount of purified IBD virus obtained may
not have provided sufficient material or detection of all structural polypeptides. As shown
in Table 1, the molecular weights of the IBD
virus proteins compared more closely with
those reported for the human rotavirus. However, the major polypeptide species (P3 and
P4) of IBD virus did not correspond with
those major proteins of the human rotavirus,
nor did IBD virus have an eighth polypeptide
of molecular weight 88Xl0". Our results concerning the similarity of its protein composition to the rotavirus group suggest its tentative inclusion into the Reov_iridae family.
Some characterization of
immunosuppression in
chickens by IBD virus1·5 ,si
This disease is of interest immunologically,
since the function of the bursa-dependent
lymphoid system is affected in young chickens.
The mechanism of the immunosuppression is
not fully understood, but presumably it results
from the loss of immunocompetcmt lymphocytes. The present studies were undertaken
to compare sequential changes in the proportion of B- and T-lymphocytes and measure
serum immunoglobulin concentrations in
chickens infected with IBD virus at different
ages. The results obtained are s ummarized
as follows . Chickens were infected with IBD
virus in ovo or at different t imes posthatching to 6 weeks of age. The B- and T-cell
responses in the lymphoid tissues and blood
were examined sequentially to 8 weel<S of age
by using indirect immunofluorescence. The
proportion of B-cells was consistently bwer
in infected birds t han in controls, especially
in chicks infected at embryos or at 1 clay old .
The proportion of T-cells increased following
these early infections but was slightly lower
in spleen and blood of bi rds infected at 1, 4,
and 6 weeks of age. Serum lgM levels dropped significantly after infection, regardless
of the time of infection. lgG levels decreased
following early infection but increased after
infection at 1 week old or more. The res ults
strongly suggest that B-cells are the target
for IBD virus infection.
Replication of IBD virus in
cultured lymphocytes(I>
Hitchner reviewed studies on laboratory
host systems for IBD virus and noted that the
virus replicates in chicken embryos and that
embryos-adapted virus could be cultured in
cell cultures of chicken embryo origin, with
consequent cytopathic effects. No published
50
information is available on replication of
virulent, nonadapted strains of the virus in
cultured lymphocytes. It has been shown in
a previous section that a specific lymphocyte
type might serve as the target for IBD virus
replication in vivo. As part of an investigation of the mechanism by which this virus
induces immunosuppression, it was of interest
to study virus replication in vitro. The results obtained are summarized as follows.
The in vitro susceptibility of chicken lymphocytes to a wild strain of IBD virus was
investigated by using immunofluorescence and
virus assays as infection criteria. A variety
of Marek's disease Jymphoblastoid cell lines,
all of thymus (T-cell) origin, were refractory
to virus exposure. However, a bursa (B-cell)derived lymphoblastoicl cell line from an avian
leukosis virus-induced tumor was highly susceptible. Viral antigen appeared in the cytoplasm of 20 to 30% of the cells, and large
amounts of cell-free virus were released, with
maximum yields occurring by 3 days postinfection. The virus also replicated in a small
percentage of normal lymphocytes prepared
from lumphoid tissues and peripheral blood
of chickens. Pretreatment of the lymphocytes,
with heat-inactivated anti-B-cell serum or
with antiserum against fowl immunoglobulin
M before inoculating them with the virus
blocked the virus infection; no blocking occurred with anti-T-cell serum or with specific
antiserum against fowl immunoglobulin G ot·
immunoglobulin A. This suggests that surface immunoglobulin M-bearing B-lymphocytes were the target cells for infection.
Pathogenicity of IBD virus in
mouse, rat and hamster0 ·10>
Rinaldi et aJ.m and Petek et al.1 6) reported
that egg-adapted strain of IBD virus was
pathogenic for suckling mice. Very little is
known of the pathogenicity to laboratory
animals. The results obtained are summarized
as follows. The susceptibility of mice and
rats became lower according with the advance
JARQ
Vol. 14, No. 1, 1980
in age regardless of inoculation routes. Mice
and rats were the most susceptible to intracerebral inoculation. T he infectivity titers in
the brains were the highest regardless of the
routes of virus inoculation. The tite1· in the
brains increased logarithmically from 12 to
60 hrs postinoculation. A plateau of 10°,8 to
10··· PFU/0.2 ml was maintained between 60
to 72 hrs. It is also noted that precipitating
antigen was produced in the brain of mice and
rats inoculated with the virus. The antigen
formed 2 or 3 precipitin lines against specific
antiserum. The principal changes of the
brains were degeneration and destrnction of
the nerve cells in theramus mid cortex. A
specific fluorescence was found in the cytoplasm of nerve cells. The virus particles were
observed in the cytoplasm of the infected
nerve cells. Suckling hamsters inoculated intracerebrally with the virus developed listlessness and marked weight loss begining on
postinoculation days 12 to 14. The occipital
curvature of the skull became prominent at
17 to 19 days after inoculation. All had mild
to severe hydrocephalus. The gross hydrocephalus was first seen at day 14 and reached
a maximum on the 21 day.
References
l) Hirai, K. et al.: lmmunodepressive effect on
infectious bursal disease virus in chickens. Avian
Dis.• 18, 50- 57 (1974).
2) Hirai, K., Shimak ura, S. & Kawamoto, E. :
Electron microscope observation of infectious
bursal disease virus. Avian Dis., 18, 467- 471
(1974).
3) Hirai, K., Kawamoto, E. & Shimakura, S.: Some
properties of precipitating antigens associated
with infectious bursa( disease virus. Infect. Jmmun .• 10, 1235- 1240 (1974).
4) Hirai, K. & Sh imakura, S.: Structure of infectious bursal disease virus. ]. Virol., 14, 964- 974
(1974).
5) Hirai, IC, Kunihiro, K. & Shimakura, S. : Characterization of the immunosuppression of chickens
by infectious bursal disease virus. Avian Dis.,
23, 950- 965 (1979).
6) Hirai, K. & Calnek, B. W. : In vitro replication of
infectious bursal disease virus in established
lymphoid cell lines and chicken B lymphocytes.
Infect. Jmmun., 25, 964-970 (1979).
51
7) Hirai, K., Kato, N. & Shimakura, S. : Further
morphological characterization and structural
proteins of infectious bursal disease virus. ] .
Virol., 32, 323- 328 (1979).
8) Hirai, K. & Funakoshi, H.: Sequential changes
of surface immunoglobulin of B cells in infec.
tious bursal disease virus-infected chickens
(Unpublished data).
9) Kawamoto, E. et al. : Growth of infectious bursa!
disease virus in suckling mice and rats. The
81st Meeting of the Jap. Soc. Vet. Sci. (1976)
[In Japanese) .
JO) Kawamoto, f:. et a l. : Hydrocephalus of suckling
hamster by infectious bursal disease virus. The
84th Meeting of the Jap. Soc. Vet. Sci. (1977)
[In Japanese).
J 1) Luftig, R. B. et al. : An ultrastructural study of
virions and cores of revirus type 3. Virology
48, 171- 181 (1972).
12) Martin, M. L., Palme1·, E. L. & Middleton, P.
J. : Ultrastructure of infantile gastroenteritis
virus. Virology, 68, 146- 153 (1975).
13) Nick, H., Cursiefen, D & Becht, H. : Structural
14)
15)
16)
17)
18)
and growth characteristics of infectious bursa!
disease virus. ]. Virol., 18, 227-234 (1976).
Palmer, E. L. & Martin, M. L.: T he fine struc
ture of the capsid of reovirus type 3. Virology,
76, 109- 113 (1977).
Palmer, E. L., Martin, M. L. & Murphy, F. A. :
Morphology and stability of infantile gastroen·
teritis virus: comparison with reov irus and blue,
tongue virus. ]. Gen. Virol., 35, 403- 414 (1977).
Petek, M., D' Aprile, N. & Cancellotti, f.: Bio·
logical and physico-chemical properties of the
infectious bursa! disease virus (IBDV). Avian
Path., 2, 135- 152 (1973).
Rinaldi, A. et al.: Untersuchungen uber die
Atiologie der sogenannten Gumboro-Krankheit.
II. Pathogene Wirkung des Virus auf einige
Laboratoriumstiere. 4th Congress of the World
Veterinary Poultry Association, Belgrade, 309-3 13
(1969).
Stannard, L. M. & Schoub, B. D. : Observations
on the morphology of two rotaviruses. ]. Gen.
Virol., 37, 435-439 (1977).
A New in vitro Method for Estimating
Digestibility of Animal Feeds
By
SHU
FU RUY A
Department of Nutrition, National Institute of Animal Industry
Digestibility experiments have a considerable value in the estimation of nutritive
values of animal diets. However, the detel'mination of digestibility is not only tedious
and time-consuming but also requires large
quantities of diets.
Recently, the author proposed a new in vitro
method to estimate the digestibility of diets
for pigs. The method is based on a simulation
of gastric followed by intestinal digestion.
Test substances (feed) are first incubated
with acid pepsin followed by incubation with
intestinal fluid obtained from a pig fitted with
a simple cannula in the upper jejunum. This
method was also applied to estimate the
digestibility of poultry diets, and activity
changes of the intestinal fluid when the fluid
was lyophilized were examined.
The present paper reports results of study
on the in vit1·0 method for the estimation of
digestibility using the intestinal fluid of the
pig.
Standard procedures in
in vitro digestion
The method had two stages: In the first
stage, 0.5 g each of duplicate samples of each
diet was weighed into 100 ml Erlenmeyer
flask, to which 20 mg pepsin in 10 ml 0.075
M-hydrochloric acid was added and incubated
with shaking at 80 oscillations/min for 4 hrs
at 37°C in a wate1·-bath. At the end of the
first incubation period, the content was
neutralized with 0.2 M-sodium hydroxide. In
the second stage, 10 ml of intestinal fluid was
added and the digestion mixture was incu-
bated for an additional 4 hrs at 37°C.
A female pig weighing approximately 25 kg
was used as a host animal to obtain the intestinal fluid for in vitro digestion experiments. The pig was fitted with a simple ('T'shaped) cannu la in the upper jejunum 500 mm
beyond the pylorus and distal to the common
bile duct. Approximately 500 g of intestinal
contents was removed daily between 10.00 and
11.00 a.m. through the cannula and centrifuged for 10 min at 1250 or 1500 g. The
supernatant fraction (intestinal fluid) was
used immediately or stored at -20°C for in
vitro digestion experiments.
At the completion of the second incubation
the content of the flask was transferred to
120 ml centrifuge-tube and centrifuged immediately for 10 min at 1250 g. The supernatant was resuspended in 50 ml water and
recentrifuged for 10 min at 1250 g. The second supernatant fraction was discarded. The
insoluble r esidue in the tube was mixed with
a little water and filtered through a weighed
filter paper. The paper containing the residue
was dried for 5 hrs at 105°C and transferred
to a Kjeldahl flask for determination of crude
protein (CP). The digestibilities of dry matter ( DM) and CP were calculated :
1--
R
s
where R is the weight of the oven-dry sample
residue and S is the weight of the sample
for each constituent.
For the in vitro determinations each diet
was ground in a laboratory mill with a 0.5
(poultry diets) or 1 (pig diets) mm screen.