* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download CytoFactors - What is anti
Survey
Document related concepts
Stem-cell therapy wikipedia , lookup
Xenotransplantation wikipedia , lookup
DNA damage theory of aging wikipedia , lookup
Aging and society wikipedia , lookup
Neurodegeneration wikipedia , lookup
Regeneration in humans wikipedia , lookup
Epigenetic clock wikipedia , lookup
Progeroid syndromes wikipedia , lookup
Immortality wikipedia , lookup
Calorie restriction wikipedia , lookup
Strategies for Engineered Negligible Senescence wikipedia , lookup
American Academy of Anti-Aging Medicine wikipedia , lookup
Gerontology wikipedia , lookup
Aging brain wikipedia , lookup
Successful aging wikipedia , lookup
Transcript
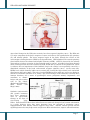
LLOYD WRIGHT’S ANTI AGING Scientific Backgrounder Douglas Laboratories Inc. All Rights Reserved XTRA-CELL LLOYD WRIGHT’S ANTI AGING™ Table of Contents Scientific Review ___________________________________________________ 3 An aging world_____________________________________________________________3 What is aging? _____________________________________________________________4 Why do we age? ____________________________________________________________4 Immunity as a key to aging _________________________________________________4 Hormones as a key to aging _________________________________________________6 Free radicals as a key to aging ____________________________________________8 Proteolytic activity as a key to aging _________________________________________11 Conclusion ________________________________________________________________13 References_________________________________________________________________13 Page 2 XTRA-CELL ANTI-AGING SUPPORT™ Scientific Review An aging world With the new millennium, population aging has been emerging as a preeminent phenomenon. The combined effects of lowered fertility rates, improved health care and longevity, better educational and nutritional status, and sustained economic development, are causing a dramatic increase in the numbers and proportions of older inhabitants throughout the world. By the year 2030, the proportion of people aged 65 and over is expected to be 13% or more in most countries. As the World War II baby-boomers begin to reach their elder years by 2010, we should see a dramatic increase in the annual percent growth of elderly population in both developed and developing countries. From 2010, this growth rate should remain level until the year 2030 when it is predicted to drop to eventually follow the steadily declining growth rate of the general population. Because the proportion of older people is increasing, there is a new pressure to better comprehend their needs as a group. Understanding the dynamics of aging requires an adequate description of the underlying physiology and biology. Page 3 XTRA-CELL ANTI-AGING SUPPORT™ What is aging? Aging is a continuous process resulting from the accumulation of little random changes affecting structural and functional elements within the body. At a molecular level, damages accumulate with time on DNA, proteins and lipids as they overcome the intrinsic repair mechanisms of the body. This build-up of molecular changes eventually affects physiological processes leading to the point where it may even compromise the general homeostasis of the body. As a consequence, we tend to become more vulnerable to environmental stress and age-related diseases as we grow old. Aging is also associated with a slower metabolism, a decrease in the number of active cells and an increase in their mutation rate. Why do we age? Although genetic disorders and gene mutations may contribute to premature aging, aging itself is not a genetically programmed process. Instead, it appears to arise indirectly through evolutionary neglect. In other words, the natural selection pressure applies until an organism actively starts reproducing itself and wanes thereafter. According to the theory of natural selection, genes that confer a better chance for survival and procreation will be passed along through generations while those that cut life short will not have that chance. But what if the deleterious trait only manifests itself in later life? Likely, it will be transmitted to the offspring. This is well illustrated by Huntingdon disease, a genetically programmed degeneration of neurons in the brain that only strikes when people are in their 30s or 40s, a time when most already had their children. Could it be that aging itself is a late onset disorder beyond the control of natural selection (Partridge and Gems, 2000; Rose, 1991)? If this is really the case, then there might be a chance to cancel the program of body aging (Skulachev, 2010). Artificial selection experiments, done on the tiny drosophila, support the evolutionary theory of aging. When eggs laid by older flies were selectively picked for further development and the process repeated over several generations, it was possible for researchers to double the lifespan of these flies. Interestingly, long-lived flies were revealed to be "superflies" that remained vigorous longer and resisted environmental stress better (Rose and Nussbaum, 1994). These results suggest that aging might be postponed by increasing the force of selection at later ages. Of course, applying such an approach to human is unthinkable given the ethical problems that it would raise. The solution rather lies in digging into molecular and physiological mechanisms of aging in the hope of mimicking the beneficial effects of artificial selection. Thanks to research, a few clues about the human ageing process have emerged over the years. Immunity as a key to aging The immunological theory of aging arose from the fact that immune functions decline with age. Foreign antigens are progressively less well recognized while, on the opposite, increased reactivity toward auto-antigens is generally observed. Thymus involution, largely due to degradation of its microenvironment, is believed to play a major role in immune senescence (Manley et al., 2011). The thymus is a gland located in the upper part of the mediastinum, behind the sternum and above the heart. The thymus produces peptide factors that contribute to the maturation of T-lymphocytes first produced in the bone marrow. T-cells are responsible for identifying foreign antigens, boosting B cells to produce appropriate antibodies, stimulating phagocytosis and eliminating cancer cells as they appear. Moreover T-cells should do all these tasks while preserving the organism's healthy cells. A good healthy thymus is vital for the development of T-cells to their full bloom and particularly to get rid of the auto-reactive lymphocytes that form every now and then. Unfortunately, starting at puberty, as we grow older the thymus goes smaller. By age 30, the thymus gland has typically decreased its mass by two-thirds and its T-lymphocytes content by 90%. By age 60, functional thymic tissue has almost completely disappeared. Concomitantly, the level of thymic hormones declines in blood as we get older (Iwata et al., 1981). The gradual loss of thymic Page 4 XTRA-CELL ANTI-AGING SUPPORT™ functions leads to an increased susceptibility to infections, cancer and autoimmune diseases as we age (Goya et al, 2002). Concomitantly with thymus involution, the nature of the T-lymphocyte response in the aging body progressively derives from a cellular-prone (Th1) to a humoral-prone (Th2) type (Ginaldi et al., 1999). Upon activation and under the influence of specific factors called cytokines, T-helper cells differentiate into either Th1 or Th2 cells. Th1 cells provide cellular protection against invading pathogens such as bacteria or virus. They also activate other immune cells responsible for tracing and eliminating cancer cells as they form. An excessive Th1 response may lead to autoimmune diseases such as type I diabetes, multiple sclerosis or rheumatoid arthritis (among others). For their part, Th2 cells fight larger parasites by promoting the production of neutralizing antibodies. Overactive Th2 cells may cause allergic responses in predisposed individuals. Th1 and Th2 cells influence each other through the production of cytokines. An overactive Th1 response blunts the Th2 arm of immunity, and vice-versa. A balanced Th1-Th2 immune responsiveness is a guarantee of good health. Unfortunately throughout life a number of events can affect the system. As an example, stress stimulates the production of cortisol, a hormone that favors Th2 response, making affected people more susceptible to viral infections. As mentioned above, age also impacts on the Th1-Th2 balance. In small children, as their immune system builds up, a Th2 type predominates. Middle-aged people tend to have a Th1 bias that switches again to a Th2 type in the elderly. As a consequence of this imbalance, aging people are more susceptible to infections such as influenza and pneumonia, old infections can be reactivated causing problems such as shingles or tuberculosis and cancer cells tend to escape immune surveillance more easily. Page 5 XTRA-CELL ANTI-AGING SUPPORT™ To thwart immune imbalance, dietary supplements containing thymus extract can be used. Thymus extract contains small peptides and other thymus-derived factors which help to restore and support an optimal immune balance (Hannappel and Huff, 2003). Thymic peptides and factors include thymopoietin, several thymosins, thymulin, thymostimulin, and thymic humoral factors. In vitro, thymus extract has the ability to stimulate maturation and differentiation of peripheral blood mononuclear cells (PBMC) in order to mount a full immune response and, in this aspect, is more powerful than Echinacea, a popular herb often used as an immunostimulant. Clinically, thymus extract supplementation has been shown to be extremely effective in treating a wide variety of illnesses (including cancer) with impaired immunological functions as a common denominator (Ioannou et al., 2012; Ben-Efraim et al., 1999; Walker, 1994 & 1998). The pertinence of thymus extract supplementation in restoring immunocompetence in the elderly is supported by studies that examined different uses of thymic peptides. In a double-blind placebo-controlled clinical study, the deemed antibody response in older men was shown to be enhanced by augmentation with thymosin (Gravenstein et al., 1989). Similarly, thymopoietin, which is yet another thymic peptide, was shown to enhance the impaired lymphocyte stimulation in older people (Verhaegen et al., 1981). In another study, herpes zoster (shingles) was used as a clinical model to study the effects of thymus extract in 28 otherwise non immunocompromised patients. Results of this double blind study reported an accelerated rate of wound healing, shorter duration of vesicles, shorter time to first and crusting lesions, and a greater reduction of pain during the acute phase (Skotnicki 1989). Hormones as a key to aging Other experiments have look at the way hormones may affect the aging clock. Menopause and andropause are the direct results of the fall in sexual hormones that takes place as we age. Other hormones such as dehydroepiandrosterone (DHEA), melatonin, thyroid and various growth hormones also decline with age, affecting among others muscle functions. Levels of neurotransmitters (dopamine, GABA, norepinephrine, acetylcholine and serotonin) also diminish in the aging brain and are at the root of some of the pathologies seen in aging such as Parkinson’s disease. On the other hand, levels of cortisol, a glucocorticoid steroid hormone, tend to rise with age. Cortisol strongly impacts on brain functions and also modulates the immune system. Sustained high cortisol levels are known to contribute to the deterioration of brain functions and immunological perturbations that are commonly seen in the elderly (Bauer et al., 2009). So, although it is not always clear if changes in hormone levels are a cause or a consequence of aging, their fluctuation obviously is responsible for some of the undesirable signs of aging. Page 6 XTRA-CELL ANTI-AGING SUPPORT™ Age-related changes in the HPA axis reactivity have been reported (Aguilera, 2011). The HPA axis is a major component of the body’s response to stress and refers to the hypothalamus (H), pituitary (P), and adrenal glands. The stress response begins in the brain, through the release of the corticotropin releasing hormone (CRH) by the hypothalamus. CRH stimulates the anterior pituitary gland which releases the adrenocorticotrophic hormone (ACTH). ACTH is detected by the adrenal cortex where it induces secretion of glucocorticoids such as cortisol. A feedback loop exists through which cortisol prevents excessive secretion of CRH, ACTH and so, refrains its own production. There is evidence that an impairment of this feedback loop in the elderly is most probably caused by a diminished sensitivity of the brain corticosteroid receptors (Aguilera, 2011). At the same time, DHEA levels (which normally buffer excess cortisol) fall, leaving the high cortisol levels unchecked (Goncharova and Lapin, 2002). The ratio of cortisol/DHEA increases with age, and is even higher in instances of dementia (de Bruin et al., 2002). Age-related changes in the HPA axis reactivity affect immune functions, are a source of psychological stress, precipitate memory impairment, and contribute to chronic fatigue and pain as often seen in the elderly (Buford and Willoughby, 2008; Ferrari and Magri, 2008) Synthetic corticosteroids and growth hormones have been tentatively used as replacement therapy in the hope of rejuvenating aging bodies. Some beneficial effects have indeed been seen with such therapies but hormone substitution is a tricky business given that many hormones have the potential of promoting cancer. Supplementation with adrenal extracts appears much safer. Adrenal extracts provide natural signalling factors that may help restore and preserve HPA axis functions, improve adrenal functions, Page 7 XTRA-CELL ANTI-AGING SUPPORT™ and maintain a proper corticosteroid balance (Wilson, 2000). Adrenal extract could benefit people experiencing age-related symptoms such as fatigue and cognitive impairment. Free radicals as a key to aging The free radical hypothesis of aging has gained in popularity among scientists, over the years (Gilca et al., 2007; Viña et al., 2007). Free radicals are molecules that have lost an electron due to the splitting of a molecular bond. Free radicals such as superoxide (.O2-) and hydroxyl radical (.OH-) are short-lived but highly reactive molecules that, in a desperate attempt to restore their integrity, will steal electrons from surrounding molecules. This ignites a chain reaction where molecules are stealing electrons from each other. Under normal conditions, the antioxidant defence system within the body, comprising superoxide dismutase (SOD), catalase and glutathione peroxidase, can handle free radicals as they are produced. But as we age, this natural defence mechanism loses its strength and free radicals are left unchecked, generating important oxidative damages to DNA, lipids and proteins in our cells that cumulate to impair bodily functions. Within cells, mitochondria are the organelles most susceptible to free radical action (GomezCabrera et al., 2012). Mitochondria, little bean-shaped organelles delimited by a double membrane, are found inside every cell and are responsible for energy production through the respiratory chain. The respiratory chain refers to an assembly of enzymes embedded within the mitochondrial membranes that work in concert to transport electrons (extracted from food) through a series of complex oxydoreduction reactions. The rate of respiration is determined by the electron flux through the respiratory chain. Electron transport is coupled to the pumping of protons that build up within the mitochondrial intermembrane space to create a pH gradient. This gradient serves as a potential energy store that is used by ATP synthase to drive the synthesis of ATP, the major source of energy for the body. In the process of energy production, mitochondria generate an important part of the free radicals susceptible of causing cellular damage. For their own protection, mitochondria have evolved specific antioxidant defences that unfortunately may become less efficient with age, resulting in the accumulation of mitochondrial damages. Old mitochondria consume less oxygen, have stiffer membranes, transport electrons much less efficiently then younger ones, generate more radicals and produce less ATP (Lee and Wei, 2012; Shigenaga et al., 1994). Mitochondrial decay has been associated with age-related conditions resulting from neuronal degeneration and decreased cell-mediated immunity (Bertoni-Freddari et al., 2004; Liu et al., 2002). Halting age-related oxidative damage to mitochondria and optimizing mitochondrial functions therefore appear as a highly desirable target in the elderly (Liu and Ames, 2005). Page 8 XTRA-CELL ANTI-AGING SUPPORT™ Apparently, caloric restriction (CR) can do just that (Speakman and Mitchell, 2011). Somehow, limiting the amount of calories available from feeding seems to force mitochondria to work more efficiently by optimizing mitochondrial respiration rate (Merry, 2002). In the process, CR limits the rate of free radical production in mitochondria, alleviating the burden of oxidative stress to cells (Liu and Ames, 2005; Lopez et al., 2002). Moreover, CR generally up-regulates the expression of genes involved in free radical scavenging, energy metabolism and genomic stability, although with some variability among tissues and species (Lee et al., 1999). This translates into direct general health benefits. In animals, CR can prevent or reduce the occurrence of age-related conditions such as heart disease, diabetes, cancer, cataracts, Parkinson’s and Alzheimer (Liu and Ames, 2005). CR can extend both the average and maximum lifespan of all animals tested ((Speakman and Mitchell, 2011; Weindruch and Walford, 1988). When applied to mice from one month of age, CR had a dramatic effect on life extension and this effect was increasing with the degree of CR, prolonging life up to 40-50%. Life extension is more modest when CR is onset later in the animal life and requires an extended gradual phase-in. Still a 10-20% increase was observed when CR was applied to animals of age equivalent to 30-40 years of human life. Page 9 XTRA-CELL ANTI-AGING SUPPORT™ CR is, in fact, the only one intervention that has ever been proven to extend the lifespan of all animal species tested. Sounds appealing? Think twice, because caloric restriction is more than just dieting. Under caloric restriction, laboratory animals are fed at least 30% fewer calories than they would normally consume to satisfy their hunger. Beyond the obvious mental distress that may result from perpetual hunger, there are other major drawbacks to CR. Rodents fed on a restricted diet are less fertile, develop a reduced muscular mass, are more susceptible to bacterial infection, heal their wounds more slowly, and suffer/shiver constantly from cold. And what about CR in humans? Short term CR experiments with humans within the Biosphere 2 project, the self-sustaining greenhouse in Arizona, resulted in substantial weight loss, remarkable fall in blood cholesterol, blood pressure, fasting blood sugar, and low white blood cell counts--exactly as seen in rodents on such a regimen (Walford et al., 1995). Potential risks with CR in humans are a decrease of stress responses and other defence mechanisms, and possibly an increased risk of osteoporosis, since lower bone densities were reported in other primates such as monkeys (Black et al., 2001). Humans on long term restriction report similar negative side effects to those observed in animals – perpetual hunger, reduced body temperature leading to a feeling of being cold, and diminished libido (Speakman and Mitchell 2011). It should be emphasized that, when transposed to a human scale, CR represents a restricted daily intake of about 600 and 800 calories for a middle-aged healthy woman and man respectively (when the recommended daily intake is normally of 1900 and 2700 calories). Strict balanced proportions between fat, proteins and carbohydrates in the diet must be respected and minerals and vitamins have to be supplemented. Clearly, this is not the kind of regimen that can be improvised and a professional follow-up with a nutritionist would be mandatory. Moreover the regimen would have to be maintained life-long for lasting benefits. Keeping up with such a Spartan regimen is not given to everybody. Could there be an easier choice? Supplementation with mesenchymal extract might be the answer. Proliferation (Hoescht DNA count) Mesenchymal extract is prepared from mammal extra-embryonic connective tissue. Mesenchymal cells are undifferentiated cells (or stem cells) that, when triggered under appropriate conditions, can become almost any type of cells to help restore damaged or aging tissues (Jamnig and Lepperdinger 2012; Caplan, 1994). Mesenchymal extract is obtained by breaking down mesenchymal stem cells to liberate active molecules. These active molecules are then selectively picked up to obtain a 250% mesenchymal liquid extract that provides a natural rich source of cell factors and signalling 200% molecules. Mesenchymal extract is known for enhancing the healing and repair of damaged or 150% slow-to-heal tissues. Moreover, and of major interest for anti-aging applications, addition of 100% mesenchymal extract to fibroblasts in culture triggered an increase in the aerobic metabolic 50% activity (respiration) of the cells, while negligibly affecting their proliferation rate. 0% FBS Serum FBS Serum + This biological profile suggests that mesenchyme Mesenchym e extract can increase mitochondrial respiration efficiency and possibly stimulate ATP production. Who would not welcome more energy? Moreover, as seen with caloric restriction, improved mitochondrial functions are associated with a slowdown of the aging process and a better recovery of oxidative insults (Broderick et al., 2002). Thus, the biological activity profile of mesenchymal extract supports its use as a nutritional supplement to regenerate functional tissues and boost energy level. Page 10 XTRA-CELL ANTI-AGING SUPPORT™ Proteolytic activity as a key to aging Proteins such as collagen, elastin and others are linked with surrounding macromolecules such as glycosaminoglycans to form the extracellular matrix (ECM) components of all solid body tissues. Examples include cartilage, the fibrous sheaths of muscles, tendons and ligaments, and the dermal layer of the skin. ECM components are in constant renewal, or turnover, a process based on equilibrium between synthesis and degradation. Collagen fibbers are synthesized by cells such as articular cartilage chondrocytes or skin fibroblasts. In addition to their anabolic activity, chondrocytes and fibroblasts also produce and secrete enzymes known as collagenases or matrix metalloproteinases (MMPs). MMPs represent a special class of enzymes that target and cleave the fibrous proteins of the ECM. Almost paradoxically, chondrocytes and fibroblasts also produce MMP inhibitors. These inhibitors are important in restraining the proteolytic action of MMPs. A balance must therefore exist between these two opposing cellular activities in order to maintain cartilage and tissue matrix integrity (Ra and Parks 2007). With aging and in various conditions, the metabolic activity of cells such as chondrocytes and fibroblasts may become disturbed. Such a disturbance may affect the equilibrium between production and breakdown of extracellular matrix (ECM) components. In these conditions, the balance between MMPs and MMP inhibitors is no longer respected so that, in most cases, the enzymatic activity of MMPs predominates. Aging, long-term sunlight exposure and mechanical stressing of joints are all factors associated with an increased enzymatic activity of MMPs (Hernández-Pérez and Mahalingam, 2012; Quan et al., 2009; Tanaka et al., 1998). This activity eventually leads to the degradation of the collagen fibre meshwork. The phenomenon is well exemplified by the fate of skin with aging. Loss of skin elasticity, wrinkling, sagging, atrophy, and changes in tone and moisture levels are hallmarks of time that have been related to excessive MMP activity (Sárdy, 2009; Quan et al., 2009). Page 11 XTRA-CELL ANTI-AGING SUPPORT™ But MMP action is more than skin deep. MMPs also influence important cellular processes and immune cell functions through proteolytic processing and shedding of bioactive molecules such as cytokines and growth factors (Vargová et al., 2012). MMPs are overactive in numerous models of inflammatory diseases and degenerative processes, including Alzheimer, Parkinson’s disease, macular degeneration, colitis, rheumatoid arthritis, osteoarthritis, periodontitis, psoriasis, asthma, chronic obstructive pulmonary disease, emphysema, multiple sclerosis, amyotrophic lateral sclerosis, atherosclerosis, arterial restenosis after angioplasty and, aneurysm development ((Hernández-Pérez and Mahalingam, 2012; Vargová et al, 2012; Tayebjee et al., 2005; Dove, 2002). How can we control MMPs? Liquid cartilage extract (LCE), contains natural molecules that can stop the proteolytic activity of MMPs. Although the exact molecular mechanism by which cartilage extract performs this inhibitory action remains elusive, it has found applications in various conditions involving MMP over activity that tend to develop with age such as cancer, psoriasis, arthritis and macular degeneration (Brown, 2000; Gingras et al., 2003; Sauder et al., 2002; Thibodeau and Behr, 2002). LCE causes anti-inflammatory action. This has been demonstrated by the effects that the extract displayed in a murine model of contact hypersensitivity in which it decreased production of several inflammatory cytokines, including interferon-gamma (INF-gamma), and stimulated production of the anti-inflammatory cytokine IL-10 (Dupont et al., 2003). Anti-inflammatory action and analgesic effects of LCE have also been demonstrated in the paw edema model in rats (Fontenele et al., 1996). Since inflammation is an important component of many age-related diseases, taming the process might be beneficial for successful aging. In addition, LCE is already widely used in cosmetic preparations as an anti-aging agent to help prevent formation of wrinkles. Maintaining a proper balance between collagen formation and degradation with LCE supplementation may help fighting the signs of age arising both internally and externally. Page 12 XTRA-CELL ANTI-AGING SUPPORT™ Conclusion Advances in knowledge and technology have generated a dramatic bound in life expectancy, which, in the United States, went from roughly 47 years in 1900 to 76.9 years in 2000. Since more people are now reaching old age, aging manifestations and limitations have become much more of a concern. Not only do we want and can live longer but we also wish to do it gracefully. A better understanding of the aging process has given us some clues on how to age well. A proper diet combined with exercise is mandatory. Supplementation with glandular extracts may be helpful in restoring and maintaining the homeostasis of the body. Glandular extracts contain concentrated active cell factors and signalling molecules derived from their tissue of origin. Thymus gland extract may help balance and boost a weakening immune system. Adrenal extract may normalize the HPA axis that is often compromised in the elderly. Mesenchymal extract provides rejuvenating molecules and can boost the energy level of cells. Cartilage extract with its anti-MMP action preserves the integrity of the extra-cellular matrix in cartilage, tendons, skin and other tissues. These extracts have been conveniently combined in a special formulation aimed at sustaining the body functions of an aging population. References Aguilera G. HPA axis responsiveness to stress: implications for healthy aging. (2011) Exp Gerontol. Feb-Mar;46(2-3):90-5. Bauer ME, Jeckel CM, Luz C. The role of stress factors during aging of the immune system. (2009) Ann N Y Acad Sci. Feb;1153:139-52. Ben-Efraim S, Keisari Y, Ophir R, Pecht M, Trainin N, Burstein Y. (1999) Immunopotentiating and immunotherapeutic effects of thymic hormones and factors with special emphasis on thymic humoral factor THF-gamma2. Crit Rev Immunol. 19(4):261-84. Bertoni-Freddari C, Fattoretti P, Giorgetti B, Solazzi M, Balietti M, Di Stefano G, Casoli T. (2004) Decay of mitochondrial metabolic competence in the aging cerebellum. Ann N Y Acad Sci. 2004 Jun;1019:29-32. Black A, Allison DB, Shapses SA, Tilmont EM, Handy AM, Ingram DK, Roth GS, Lane MA. (2001) Calorie restriction and skeletal mass in rhesus monkeys (Macaca mulatta): evidence for an effect mediated through changes in body size. J Gerontol A Biol Sci Med Sci. Mar;56(3):B98-107. Broderick TL, Belke T, Driedzic WR. (2002) Effects of chronic caloric restriction on mitochondrial respiration in the ischemic reperfused rat heart. Mol Cell Biochem Apr;233(1-2):119-25. Brown PD. (2000) Ongoing trials with matrix metalloproteinase inhibitors. Expert Opin Investig Drugs. Sep;9(9):2167-77. Buford TW, Willoughby DS. (2008) Impact of DHEA(S) and cortisol on immune function in aging: a brief review. Appl Physiol Nutr Metab. Jun;33(3):429-33. Caplan AI. (1994) The mesengenic process. Clin Plast Surg. Jul; 21(3):429-35. de Bruin VM, Vieira MC, Rocha MN, Viana GS (2002) Cortisol and dehydroepiandosterone sulfate plasma levels and their relationship to aging, cognitive function, and dementia. Brain Cogn., Nov;50(2):316-23. Dove A. (2002) MMP inhibitors: glimmers of hope amidst clinical failures. Nat Med. Feb;8(2):95. Dupont E, Wang B, Mamelak AJ, Howell BG, Shivji G, Zhuang L, Dimitriadou V, Falardeau P, Sauder DN., (2003) Modulation of the Contact Hypersensitivity Response by AE-941 (Neovastat), a Novel Antiangiogenic Agent. J Cutan Med Surg. 7(3):208-16. Ferrari E. and Magri F. (2008) Role of neuroendocrine pathways in cognitive decline during aging. Ageing Res Rev. 2008 Jul;7(3):225-33. Fontenele JB, Viana GS, Xavier-Filho J, de-Alencar JW. (1996) Anti-inflammatory and analgesic activity of a water-soluble fraction from shark cartilage. Braz J Med Biol Res.. May;29(5):643-6. Gilca M, Stoian I, Atanasiu V, Virgolici B. (2007)The oxidative hypothesis of senescence. J Postgrad Med. Jul-Sep;53(3):207-13. Ginaldi L, De Martinis M, D'Ostilio A, Marini L, Loreto MF, Quaglino D. (1999) The immune system in the elderly: III. Innate immunity. Immunol Res. 20(2):117-26. Gingras D, Boivin D, Deckers C, Gendron S, Barthomeuf C, Beliveau R. (2003) Neovastat-a novel antiangiogenic drug for cancer therapy. Anticancer Drugs. Feb;14(2):91-6. Page 13 XTRA-CELL ANTI-AGING SUPPORT™ Gomez-Cabrera MC, Sanchis-Gomar F, Garcia-Valles R, Pareja-Galeano H, Gambini J, Borras C, Viña J. (2012) Mitochondria as sources and targets of damage in cellular aging. Clin Chem Lab Med. Feb 1;50(8):1287-95. Goya, R.G., G.M. Cónsole, C.B. Hereñú, O. A. brown and O.J. Rimoldi (2002) Thymus and aging: potential of gene therapy for restoration of encodrine thymic function in thymus-deficient animal models. Gerontology, 48: 315-328. Gravenstein, S., Duthie, E.H., Mille,r B.A., Roecke,r E., Drinka, P., Prathipati, K. and Ershler, W.B. (1989) Augmentation of influenza antibody response in elderly men by thymosin-1. A double blind placebo controlled clinical study. J. Am Geriatr. Soc. 37: 1-8 Goncharova ND, Lapin BA. (2002 ) Effects of aging on hypothalamic-pituitary-adrenal system function in non-human primates. Mech Ageing DevApr 30;123(8):1191-201. Hannappel E. and Huff T. The thymosins. (2003) Prothymosin alpha, parathymosin, and betathymosins: structure and function. Vitam Horm. 66:257-96. Hernández-Pérez M, Mahalingam M. (2012) Matrix metalloproteinases in health and disease: insights from dermatopathology. Am J Dermatopathol. Aug;34(6):565-79. Ioannou K, Samara P, Livaniou E, Derhovanessian E, Tsitsilonis OE. (2012) Prothymosin alpha: a ubiquitous polypeptide with potential use in cancer diagnosis and therapy. Cancer Immunol Immunother. May;61(5):599-614. Iwata T, Incefy GS, Cunningham-Rundles S, Cunningham-Rundles C, Smithwick E, Geller N, O'Reilly R, Good RA. (1981) Circulating thymic hormone activity in patients with primary and secondary immunodeficiency diseases. Am J Med. Sep;71(3):385-94. Jamnig A. and Lepperdinger G. (2012) From tendon to nerve: an MSC for all seasons. Can J Physiol Pharmacol. Mar;90(3):295-306. Lee CK, Klopp RG, Weindruch R, Prolla TA. (1999) Gene expression profile of aging and its retardation by caloric restriction. Science. Aug 27;285(5432):1390-3. Lee HC, Wei YH. (2012) Mitochondria and aging. Adv Exp Med Biol. 942:311-27. Liu J and Ames BN. (2005) Reducing mitochondrial decay with mitochondrial nutrients to delay and treat cognitive dysfunction, Alzheimer's disease, and Parkinson's disease. Nutr Neurosci. Apr;8(2):67-89. Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN. (2002) Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci U S A. Feb 19;99(4):2356-61. Lopez-Torres M, Gredilla R, Sanz A, Barja G. (2002) Influence of aging and long-term caloric restriction on oxygen radical generation and oxidative DNA damage in rat liver mitochondria. Free Radic Biol Med. May 1;32(9):882-9. Manley NR, Richie ER, Blackburn CC, Condie BG, Sage J. (2011) Structure and function of the thymic microenvironment. Front Biosci. Jun 1;16:2461-77 Merry BJ. (2002) Molecular mechanisms linking calorie restriction and longevity. Int J Biochem Cell Biol. Nov;34(11):1340-54. Partridge, L. and D. Gems (2002) Mechanisms of ageing: public or private? Nature Reviews [Genetics], 31: 165-175. Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ, Fisher GJ. (2009) Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc. Aug;14(1):20-4. Ra HJ, Parks WC. (2007) Control of matrix metalloproteinase catalytic activity. Matrix Biol. Oct;26(8):587-96. Rose, M.R. (1991) Evolutionary biology of aging. Oxford University Press, 221 p. Rose, M.R. and T.J. Nusbaum (1994) Prospects for postponing human aging. FASEB Journal, 8: 925-928. Sárdy M. (2009) Role of matrix metalloproteinases in skin ageing. Connect Tissue Res. 50(2):132-8. Sauder DN, Dekoven J, Champagne P, Croteau D, Dupont E. (2002) Neovastat (AE-941), an inhibitor of angiogenesis: Randomized phase I/II clinical trial results in patients with plaque psoriasis. J Am Acad Dermatol. Oct;47(4):535-41. Shigenaga MK, Hagen TM, Ames BN. (1994) Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. Nov 8;91(23):10771-8 Page 14 XTRA-CELL ANTI-AGING SUPPORT™ Skotnicki AB. (1989) Therapeutic application of calf thymus extract (TFX). Med Oncol Tumor Pharmacother., 6(1):31-43. Skulachev VP. (2010) How to cancel the program of body aging? Russian Journal of General Chemistry, Volume 80, Number 7:1523-1541. Speakman JR. and Mitchell SE. (2011) Caloric restriction. Mol Aspects Med. Jun;32(3):159-221. Tanaka S, Hamanishi C, Kikuchi H, Fukuda K. (1998) Factors related to degradation of articular cartilage in osteoarthritis: a review. Semin Arthritis Rheum. Jun;27(6):392-9. Tayebjee MH, Lip GY, MacFadyen RJ. (2005) Matrix metalloproteinases in coronary artery disease: clinical and therapeutic implications and pathological significance. Curr Med Chem. 12(8):917-25. Thibodeau, A, et S. Behr (2002) Liquid cartilage extract decreases symptoms of rheumatoid arthritis. Townsend Letter for Doctors & Patients, May: 59-62. Vargová V, Pytliak M, Mechírová V. (2012) Matrix metalloproteinases. EXS. 103:1-33. Review. PubMed PMID: 22642188. Verhaegen H, De Cock W, De Cree J, Goldstein G. (1981 ) Restoration of the impaired lymphocyte stimulation in old people by thymopoietin pentapeptide. J Clin Lab Immunol. Sep;6(2):103-5. Viña J, Borrás C, Miquel J. (2007) Theories of ageing. IUBMB Life. Apr-May;59(4-5):249-54. Walford RL, Weber L, Panov S. (1995) Caloric restriction and aging as viewed from Biosphere 2. Receptor. Spring;5(1):29-33. Walker, M. (1994) Sublingual live proteins; mesenchyme, thymus & liquid Shark cartilage. Townsend Letter for Doctors & Patients, October: 1041-1046. Walker, M. (1998) Clinical applications of frozen sublingual thymic extract. Townsend Letter for Doctors & Patients, December: 82-87. Weindruch, R. and Walford, R.L. (1988). The retardation of aging and disease by dietary restriction, C.C. Thomas, Springfield IL. Wilson, J. (2000a) Mesenchyme: little known rejuvenating healer. The Original Internist, September, 13-18. Wilson (2000b) The use of adrenal cortical extracts in adrenal fatigue. Townsend Letter for Doctors & Patients, December: 83-87. Page 15