* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 4 ppt
Gastrointestinal tract wikipedia , lookup
Toxicodynamics wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Drug design wikipedia , lookup
Pharmacognosy wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Theralizumab wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug discovery wikipedia , lookup
Drug interaction wikipedia , lookup
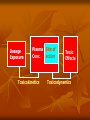
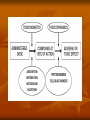
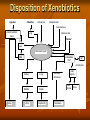
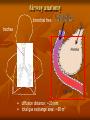
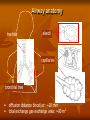
Toxicokinetics Toxicokinetics is the study of the drug movement around the body (Absorption, Distribution, metabolism, and Elimination) Toxicokinetic data is best derived using radio labeled dose of the drug. This allows for following the fate of the drug, metabolic products, distribution in the tissue, storage sites, as well as its elimination. Unfortunately, these methods do not provide knowledge about proportion of the drug left intact to its metabolites. TK is concerned with what the body does to the toxicant. TOXICOKINETICS Absorption Non-compartment Zero First Order of reaction Quantitative model Compartment Physiological-based TK Clearance Half-life Volume of distribution Bioavailability Active Passive Ingestion Inhalation Skin penetration Parenteral Parameters Distribution Circulation Adipose tissue Highly perfused ogan Blood-brain barrier Metabolism Phase 1 Phase 2 Toxicokinetics Membrane transportation Excretion Kidney Lung Feces Saliva Lactation Sweating Toxicodynamics Toxicodynamics is the study of toxic actions of xenobiotic substances on living systems. Toxicodynamics is concerned with processes and changes that occur to the drug at the target tissue, including metabolism and binding that results in an adverse effect. Simply, TD is concerned with what the toxicant do to the body Dosage Exposure Plasma Site of action Conc. Toxicokinetics Toxic Effects Toxicodynamics Toxicokinetic (TK) processes ABSORPTION xenobiotic EXTERNAL MEMBRANE BARRIERS skin G.I. tract lungs DISTRIBUTION BLOOD PLASMA TISSUES depots METABOLISM PHASE-1 PHASE-2 EXCRETION KIDNEYS LIVER lungs saliva sweat breast milk Disposition of Xenobiotics Ingestion Inhalation Intravenous Intraperitoneal Subcutaneous Gastrointestinal tract absorption Intramuscular Lung Dermal Liver Blood and lymph Bile extracellular fluid fat distribution Kidney Bladder feces Urine Lung Secretory Structures soft tissue Alveoli Expired Air body organs Secretions bone excretion Toxicokinetics (ADME) Toxicokinetics study four processes: 1. Absorption 2. Distribution 3. Metabolism 4. Excretion Metabolism and excretion processes are combined as a single process called elimination The toxicokinetics of a chemical are determined by measuring the concentrations of the chemical in plasma (usually) or blood at various times following a single dose. The fundamental parameters that define the rates and extents of distribution and elimination are derived from data following an intravenous or oral dose. Important principles of toxicokinetics 1. 2. The effect which a drug produces is dependent on: The dose The concentration in the target organ The kinetics of a drug may differ from therapeutic dose to its toxic dose Toxicokinetics is important in predicting the plasma concentration of a drug Toxicokinetics and toxicity Toxicity depends on: Duration and concentration of drug at the portal of entry The rate and amount (extent) of drug absorbed; toxicity will be low at slow absorption rates. This means that a highly toxic drug that is poorly absorbed may have same hazard as another with low toxicity but is highly absorbed. The distribution of drug within the body; where most drugs are distributed in highly perfused organs like brain, liver and kidneys. However, in some cases, the organ in which the drug is concentrated may not necessarily suffer the damage. An example is organochlorine compounds concentrated in adipose tissue while the target organ is the brain. The efficiency of biotransformation and nature of metabolites; where, in some cases, a drug may be transformed to a more toxic metabolite or a more lipid soluble or water soluble metabolite, which affects absorption and distribution The ability of the drug to pass through cell membranes and interact with cell constituents. Example, some organochlorines affect the DNA The amount and storage duration of the drug or its metabolites in the tissue. These may induce toxicity after a long time after exposure. Lead in bones is an example The rate and site of excretion; where the more rapid the excretion, the less toxicity it will produce 1. Absorption The term absorption describes the process of the transfer of the parent chemical from the site of administration into the general circulation, and applies whenever the chemical is administered via an extravascular route (i.e. not by direct intravascular injection). Many chemicals will be metabolized or transformed during their passage from the site of administration into the general circulation, so that little parent chemical may reach the general circulation, this raises the possibility of confusion in discussing the ‘extent of absorption’ depending on whether the data refer to the parent chemical, or to metabolites or both (when radiolabeling is used). This confusion is resolved by the proper use of the term bioavailability (the fraction of the dose administered that reaches the general circulation as the parent compound) to describe the extent of absorption. Mechanism of Membrane Permeation 1. 2. 3. 4. Passive diffusion Active transport Facilitated transport Pinocytosis and phagocytosis Drugs are absorbed by the following processes: Passive transport This can occur by simple diffusion due to concentration gradient or By passage of drugs through the pores (of the kidney and capillaries), i.e. by filtration Passive transport is affected by: Ability of the drug to dissolve in the lipid portion of the cell membrane The size of the drug, in case it is water soluble. Aqueous pores are about 4Ao which will allow drugs of 100-200 amu to pass Presence of the drug in its nonionized form 1. Uptake by Passive diffusion • Uncharged molecules may diffuse along conc. gradient until equilibrium is reached • No substrate specificity • Small MW < 0.4 nm (e.g. CO, N20, HCN) can move through cell pores • Lipophilic chemicals may diffuse through the lipid bilayer Uptake by Passive diffusion First order rate diffusion, depends on • Concentration gradient • Surface area (alveoli 25 x body surface) • Thickness • Lipid solubility & ionization • Molecular size (membrane pore size = 4-40 A, allowing MW of 100-70,000 to pass through) Flicks’s law and Diffusion dD/dt = KA (Co - Ci) / t Where; dD/dt = rate of mass transfer across the membrane K = constant (coefficient of permeability) A = Cross sectional area of membrane exposed to the compound C0 = Concentration of the toxicant outside the membrane Ci = Concentration of the toxicant inside the membrane t = Thickness of the membrane 2. Special transport Two types of special transport mechanisms can be identified: 1. Active diffusion: Independent of or against conc. gradient Require energy Substrate –specific Rate limited by no. of carriers Example: Ca-pump (Ca2+ -ATPase) 2. Facilitated diffusion: Occurs when a drug has a specific carrier protein, and does not occur against concentration gradient Carried by trans-membrane carrier along concentration gradient Energy independent May enhance transport up to 50,000 folds Example: Calmodulin for facilitated transport of Ca2+ 3. Additional transport: occurs by endocytosis; where : Phagocytes (cell eating) engulf the solid large particles suspended in the intracellular fluid Pinocytes (cell drinking) in which very small suspended particles or liquids are engulfed Factors affecting gastrointestinal absorption 1. Types of cells at the specific site: An example is the sublingual cells which are highly vascularized which allows for rapid absorption 2. Period of time that drugs remain at the site: Drugs are poorly absorbed within the mouth because the time a drug spends in the mouth is very short, while high absorption can occur in the intestine due to the long time a drug spends there 3. pH This factor affects the ionizability of the drug. The acidic nature of the fluid in the stomach facilitates the absorption of weakly acidic drugs, while both weakly acidic and basic drugs are well absorbed in the small intestine since the pH there is almost neutral 4. The concentration at the absorption site 5. Presence of food or binding substances: These will decrease the concentration of the free drug and thus will lower its absorption 6. Rate of gastric emptying: As emptying rate is decreased, absorption in the stomach will increase 7. Gastrointestinal motility: This will decrease the amount absorbed in the stomach while increase the amount absorbed in the intestine 8. Absorbing surface area of the intestine 9. Blood flow to the site 10. Intestinal bacteria and gastrointestinal enzyme level 11. General condition of the patient: Comatose decrease motility thus affecting absorption 12. Drug formulation: whether it is a slow release or other form Factors affecting pulmonary absorption Solubility of the drug in the blood 2. Particle size Large particles are deposited in the nasal tract > 5 microns; 2-5 micron particles are deposited mainly in the tracheabronchial region; while particles less than 1 micron penetrate into the alveolar sacs and absorbed into the blood 3. Water solubility High water solubility volatile drugs are absorbed in the nasal tract; while low water solubility drugs will reach the bronchioles to alveoli 1. Airway anatomy bronchial tree trachea • • diffusion distance: ~20 mm total gas exchange area: ~80 m2 Airway anatomy trachea alveoli capillaries bronchial tree • • diffusion distance blood/air: ~20 mm total exchange gas exchange area: ~80 m2 Factors affecting dermal absorption 1. Condition of the skin: Stratum corneum serves as the main barrier. When abraded, increased absorption will result 2. Skin permeability coefficient This represents the rate at which a particular drug penetrates the skin 3. Body region Not all regions of the body have the same skin thickness. Forehead versus palm 4. Lipid solubility The more lipid soluble the drug is the more it will be absorbed 5. Skin hydration Rate of Absorption The rate of absorption may be of toxicological importance because it is a major determinant of the peak plasma concentration and, therefore, the likelihood of acute toxic effects. Transfer of chemicals from the gut lumen, lungs, or skin into the general circulation involves movement across cell membranes, and simple passive diffusion of the unionized molecule down a concentration gradient is the most important mechanism. Lipid-soluble molecules tend to cross cell membranes easily and are absorbed more rapidly than water-soluble ones. The gut wall and lungs provide a large and permeable surface area and allow rapid absorption; in contrast the skin is relatively impermeable and even highly lipidsoluble chemicals can enter only slowly The lipid solubility and rate of absorption depend on the extent of ionization of the chemical. Compounds are most absorbed from regions of the gastrointestinal tract at which they are least ionized. Weak bases are not absorbed from the stomach, but are absorbed from the duodenum which has a higher pH, whereas weak acids are absorbed from the stomach. The rate of absorption can be affected by the vehicle in which the compound is given, because rapid absorption requires the establishment of a molecular solution of the chemical in the gut lumen. Extremely lipid soluble compounds, such as dioxins, may be only partially absorbed, because they do not form a molecular solution in the aqueous phase of the intestinal contents. Extent of Absorption The extent of absorption is important in determining the total body exposure or internal dose, and therefore is an important variable during chronic toxicity studies and/or chronic human exposure. The extent of absorption depends on the extent to which the chemical is transferred from the site of administration into the local tissue, and the extent to which it is metabolized or broken down by local tissues prior to reaching the general circulation. An additional variable affecting the extent of absorption is the rate of removal from the site of administration by other processes compared with the rate of absorption Chemicals given via the gastrointestinal tract may be subject to a wide range of pH values and metabolizing enzymes in the gut lumen, gut wall, and liver before they reach the general circulation. The initial loss of chemical prior to it ever entering the blood is termed first-pass metabolism or presystemic metabolism; it may in some cases remove up to 100% of the administered dose so that none of the parent chemical reaches the general circulation. The intestinal lumen contains a range of hydrolytic enzymes involved in the digestion of nutrients. The gut wall can perform similar hydrolytic reactions and contains enzymes that can oxidize many drugs FIRST PASS EFFECT Intestinal vs. gastric absorption Absorption and Bioavailability Irrespective of the reason that is responsible for the incomplete absorption of the chemical as the parent compound, it is essential that there is a parameter which defines the extent of transfer of the intact chemical from the site of administration into the general circulation. This parameter is the bioavailability, which is simply the fraction of the dose administered that reaches the general circulation as the parent compound. (The term bioavailability is perhaps the most misused of all kinetic parameters and is sometimes used incorrectly in a general sense as the amount of drug available specifically to the site of toxicity). Extent of Absorption or Bioavailability Destroyed in gut Not absorbed Destroyed by gut wall Destroyed by liver Dose to systemic circulation Bioavailability Definition: the fraction of the administered dose reaching the systemic circulation and is thus a measure of first pass elimination for i.v.: 100% for non i.v.: ranges from 0 to 100% e.g. lidocaine bioavailability 35% due to destruction in gastric acid and liver metabolism Liver vein Systemic circulation Liver Liver artery Plasma concentration 70 60 Bioavailability (F) (AUC)o (AUC)iv i.v. route 50 40 oral route 30 20 Time (hours) 10 0 0 2 4 6 8 10 Principle For xenobiotics taken by routes other than the iv, the extent of absorption and the bioavailability must be understood in order to determine whether a certain exposure dose will induce toxic effects or not. It will also explain why the same dose may cause toxicity by one route but not the other. Calculation of Bioavailability The fraction absorbed as the intact compound or bioavailability (F) is determined by comparison with intravenous (i.v.) dosing (where F = 1 by definition). The bioavailability can be determined from the area under the plasma concentration–time curve (AUC) of the parent compound , or the percentage dose excreted in urine as the parent compound, i.e. for an oral dose: 2. Distribution Distribution is the reversible transfer of the chemical between the general circulation and the tissues. Irreversible processes such as excretion, metabolism, or covalent binding are part of elimination and do not contribute to distribution parameters. The important distribution parameters relate to the rate and extent of distribution. Alter plasma binding of chemicals 1000 molecules 99.9 % bound 90.0 1 molecules free 100 100-fold increase in free pharmacologically active concentration at site of action. NON-TOXIC TOXIC Rate of Distribution The rate at which a chemical may enter or leave a tissue may be limited by two factors: (i) the ability of the compound to cross cell membranes and (ii) the blood flow to the tissues in which the chemical accumulates. The rate of distribution of highly water-soluble compounds may be slow due to their slow transfer from plasma into body tissues such as liver and muscle; water-soluble compounds do not accumulate in adipose tissue. In contrast, very lipid-soluble chemicals may rapidly cross cell membranes but the rate of distribution may be slow because they accumulate in adipose tissue, and their overall distribution rate may be limited by blood flow to adipose tissue The rate of distribution is indicated by the distribution rate constant, which is determined from the decrease in plasma concentrations in early time points after an intravenous dose. The rate constants refer to a mean rate of removal from the circulation and may not correlate with uptake into a specific tissue. Once an equilibrium has been reached between the general circulation and a tissue, any process which lowers the blood (plasma) concentration will cause a parallel decrease in the tissue concentration. Factors affecting distribution Blood flow Drugs are readily distributed to highly perfused tissue like brain, liver, and kidneys 2. Permeability limitations Many drugs do not readily enter the brain due to the blood brain barrier 3. Protein binding Acidic drugs are bound to the most abundant plasma protein (albumin); while basic drugs bind to a-1acid glycoprotein. 1. 4. Effect of pH The pH of the blood or tissue affect the ionization of the drug and thus its distribution 5. Age In old people, Protein binding and body water will decrease, thus increasing the concentration of the drug per unit time 6. Existence of storage sites: These include: Adipose tissue, plasma proteins, liver, kidneys, and bone Extent of Distribution The extent of tissue distribution of a chemical depends on the relative affinity of the blood or plasma compared with the tissues. Highly water-soluble compounds that are unable to cross cell membranes readily are largely restricted to extracellular fluid (about 13 L per 70 kg body weight). Water-soluble compounds capable of crossing cell membranes (e.g. caffeine, ethanol) are largely present in total body water (about 41 L per 70 kg body weight). Lipid-soluble compounds frequently show extensive uptake into tissues and may be present in the lipids of cell membranes and adipose tissue. . A factor which may further complicate the plasma/tissue partitioning is that some chemicals bind reversibly to circulating proteins such as albumin (for acid molecules) and acid glycoprotein (for basic molecules). The extent and pattern of tissue distribution can be investigated by direct measurement of tissue concentrations in animals. Tissue concentrations cannot be measured in human studies and, therefore, the extent of distribution in humans has to be determined based solely on the concentrations remaining in plasma or blood after distribution is complete. volume of distribution Chemicals appear to distribute in the body as if it were a single compartment. The magnitude of the chemical’s distribution is given by the apparent volume of distribution (Vd). Volume of Distribution (Vd) Volume into which a drug appears to distribute with a concentration equal to its plasma concentration after distribution is complete Vd = Amount of drug in body Concentration in Plasma when a chemical shows a more extensive reversible uptake into one or more tissues the plasma concentration will be lowered and the value Vd will increase. For highly lipid-soluble chemicals, such as organochlorine pesticides, which accumulate in adipose tissue, the plasma concentration may be so low that the value of Vd may be many liters for each kilogram of body weight. This is not a real volume of plasma and therefore Vd is called the apparent volume of distribution. It is an important parameter because extensive reversible distribution into tissues, which will give a high value of Vd , is associated with a low elimination rate and a long half-life . It must be emphasized that the apparent volume of distribution simply reflects the extent to which the chemical has moved out of the site of measurement (the general circulation) into tissues, and it does not reflect uptake into any specific tissue(s).