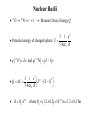* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Nuclear Structure, Nuclear Force
Two-dimensional nuclear magnetic resonance spectroscopy wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Nuclear fission wikipedia , lookup
Nuclear fusion wikipedia , lookup
Valley of stability wikipedia , lookup
Nuclear binding energy wikipedia , lookup
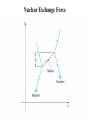
Nuclear Structure, Nuclear Force Gocha Khelashvili, Ph.D. Nuclear Structure • • • • • • • • • Composition of the Nucleus Atomic Constituents Nuclear Shape Nuclear Stability The Nuclear Force Liquid – Drop Model SEMF Binding Energy Nuclear Force Revisited Nuclear Constituents • Z - number of protons (charge of the nucleus). • The nucleus has a mass ≈ A ⋅ m p - led to believe that nucleus has A protons 1 • The nucleus has a chagre Z ≈ A 2 Is there Electron inside of Nucleus? • Before discovery of neutron it was believed that there are A − Z electrons inside of nucleus. • The existance of electron inside of nucleus was supported by the observation of β − radioactive decay, in which electrons are ejected by certain radiocative nuclei. • There were significant problems with this model. Is there Electron inside of Nucleus? • Uncertainity Principle → in order for electron to be confined in the region of r < 10−14 m, electron should have Emin ≈ 100 MeV energy. • Hovewer, energies of electrons in β decay are 1-2 MeV. • There is no evidence of force 50-100 MeV between electron and nuclei. Is there Electron inside of Nucleus? • If Ee < 0 - electron would never escape • If Ee > 0 - There is no barrier to overcome; All naturally occuring β emitters should have dissapeared from the Earth long time ago. Is there Electron inside of Nucleus? e • µ N = - measured magnetic moment of nuclei (order or nuclear magneton). 2m p e • µB = 2me - should be of order or Bohr magneton if electron is inside of nuclei. • µN 1 - measurement shows 2000 times smaller magnetic momentum ≈ µ B 2000 compared to Bohr magneton. Is there Electron inside of Nucleus? • 14 N - nitrogen nucleus - angular mometum has quantum number 1 (observed in hyperfine structure). • If 14 N containes 14 protons and 7 electrons, the resulting angular momentum would have quantum numbers 1/2, 3/2, 5/2 etc. It would be Fermion and it would obey Fermi-Dirac statistics. • But it obeys Bose-Einstein statistics. • Rutherford suggested existance of neutral particle, possibly a bound state of proton and electron and called it neutron Discovery of Neutron • 1930 W. Bothe and H. Becker - Alpha particles incident on a beryllium foil cause the emission of uncharged radiation capable of penetrating lead. • 1932 Irene Joliot-Curie and Frederic Joliot - Protons of up to 5.7 MeV are ejected when the radiation strikes a paraffin slab. They assumed that radiation consisted of gamma ray photons and the protons are knocked out of the hydrogen-rich paraffin in Compton collisions. • Using Compton’s theory they estimated energy of incident gamma rays and found that energies must be at least 55 MeV. • 1932 James Chadwick - Radiation consists of neutral particles of approximately proton mass. In this case their energies need not exceed 5.7 MeV since in head on collision between particles of the same mass all energy is transferred to target particles – protons. Composition of the Nucleus • After Chadwick's discovery of neutron the idea of neutron being a tightly bound state of proton and electron was abandoned Composition of the Nucleus • Z - number of protons (charge of the nucleus). • N - number of neutrons. • A = Z + N - Mass number of nucleus. • A particular nuclear species is called a nuclide. • Nuclides are denoted by the chemical symbol of element with presuperscript giving value of A - 16 O, 15 O,.... • Sometimes Z is given as presubscript - 158 O (not necessary - chemical symbol Z ) • Sometimes N is given as subscript - 158 O7 (not necessary - N= A − Z ) • Isotopes - same Z (protons) and different N (neutrons) - O and 15 O • Isotones - same N (neutrons) and different Z (protons) - 13 C and 14 N • Isobars - same A (neutrons + protons) - 14 C and 14 N 16 Composition of the Nucleus Composition of the Nucleus mass unit → = u 1.66054 ×10−27 kg → mass of 126C is exactly 12u Energy equivalent of mass unit → 931.49 MeV Nuclear Radii Nuclear Radii Nuclear Radii • 15 O → 15 N + e − +ν → Measure Decay Energy Q 3 1 q2 • Potential energy of charged sphere: U = 5 4πε 0 R • q ( 15 O ) = Ze and q ( 15 N ) = ( Z − 1) e 3 1 e2 2 2 •Q= ∆U = Z − ( Z − 1) 5 4πε 0 R • R =R0 A1/3 where R0 ≈ (1.2 ± 0.2 ) ×10−15 m =1.2 ± 0.2 fm Ground-State Properties of Nuclei • After 27 MeV energy of alpha particle experimental curve deviates from Rutherford formula. • Energetic alpha particle penetrates nucleus deep enough to interact directly with protons and neutrons with attractive nuclear force. • Thus scattering intensity falls. Nuclear Radii • SLAC experiment (1953) - nuclei bombarded with electrons having 200-500 MeV energies. • λ ( 500 MeV electron ) 2.5 fm (de Broglie formula) • 2.5 fm < radius of heavy nuclei • Study a structure of heavy nuclei by analyzing electron diffraction patteren. 0.61λ • First minimum - sinθ = R Nuclear Radii Example: Using the data for 420 MeV electron scattered from of 16 16 O, estimate the radius O nucleus. 0.61λ , where θ 440 = sin θ R de Broglie wavelenght of 420 MeV electron - λ = p c = E − ( mc 2 2 2 h p ) = ( 420 ) − ( 0.511) ≈ ( 420 MeV ) 2 2 2 2 2 or p = 420 MeV/c → λ = R 0.61 ⋅ 2.95 = 2.59 fm 0 sin 44 1239.8 ( eV ⋅ nm ) hc −6 = = 2.95 × 10 ( nm ) = 2.95 fm 6 420 MeV 420 ×10 ( eV ) Nuclear Size and Shape R = R0 A1/3 where R0 ≈ (1.2 ± 0.2 ) ×10−15 m =1.2 ± 0.2 fm Example: find the density of the 126C nucleus R ≈ 1.2 × (12 ) fm = 2.7 fm 1/3 m ρ = = 4 π R3 3 (12u ) (1.66 ×10−27 kg / u ) 3 4 −15 m π 2.7 10 × 3 2.4 ×1017 kg/m3 → 4 billion tons per cubic inch! ρ= ( ) The Nuclear Force – Range Behavior • Strongly attractive component which acts only over short range • At very short distances (<0.5 fm) repulsive component • Equilibrium - leads to the saturation of nuclear force. • Evidence - approximately constant density of nuclear matter. The Nuclear Force – Charge Dependence • Nuclear force is charge symmetric: n-n and p-p forces are the same in a given state • Evidence: Stability of nucleus N ≈ Z (A ≤ 40). This would not be a case if n-n p-p forces are different. • Why do we see different potentials on the picture above? Stability Curve • For A ≤ 40 → N ≈ Z for stable nuclei • For A > 40 → N > Z for stable nuclei Nuclear Stability • Ignore electrostatic repulsion between protons for light nuclei (A ≤ 40) • Energy is smallest if A/2 are neutrons and A/2 are protons • Energy is greatest if there only one type of particle (exclusion principle) The Nuclear Force – Charge Dependence • For heavy nuclei (A > 40), electrostatic repulsion between protons becomes important. • Potential energy becomes Z 2 dependent. • The energy inceased less by adding two neutrons than by adding one neutron and one proton. • N − Z increases for stable nuclei with inceasing Z . Stable Isotopes (N vs. Z) • There are about 270 stable nuclides and about 100 different elements. • 2.7 stable isotopes per element • There are larger than average number of stable isotopes with nuclei with Z equal 2, 8, 20, 28, 50, 82 and 126 (last is theoretical for now) • "Magic Numbers" - closed shell structure, very much as "magic atomic numbers" - 2, 10, 18 and 36 corresponds to closed-electron shell structure. The Nuclear Force – Charge and Spin Dependence • Nuclear force is almost charge idependent ≈ same for n-p, n-n and p-p 0 →S = ↑ 1/ 2 ↓ 1/2 p-p 0 →S = ↑ 1/ 2 ↓ 1/2 p-n Force is stronger for S 1 state. = ↑ 1/ 2 ↓ 1/2 → S 0,1 = ↑ 1/ 2 ↑ 1/2 n-n Binding Energy Mass of 11 H atom 1.007825u + Mass of neutron +1.008665u Expected mass of 21 H atom Measured mass of the 2 1 2.016490u H atom is only 2.014102u (less than expected value) = ∆m 2.016490u − 2.014102u = 0.002388u The energy equivalent of missing mass is: ∆E = ( 0.002388u )( 931.49 MeV/u ) 2.224 MeV Missing mass (energy) corresponds to energy given off when 21 H nucleus is formed or it is energy required to break apart a deuterium nucleus into separate neutron and proton Binding Energy Binding Energy per Nucleon Nuclear binding energies are strikingly high. The range for stable nuclei is from 2.2 MeV for 21 H (deuterium) to 1640 MeV for 209 83 Bi (an isotope of the metal bismuth). Typical binging energy is - 8 ×1011 kJ/kg − 800 billion kJ/kg Boiling Water - 2260 kJ/kg (heat of vaporization) Burning gasoline - 4.7 ×104 kJ/kg (17 million times smaller) Binding Energy per nucleon = ( ) = B 21 H Total Binding Energy A ( ) 1640 MeV 2.2 MeV B 209 Bi = 7.8 MeV = 1.1 MeV and for= 83 209 2 Binding Energy Curve Binding Energy Calculations Example: (a) Find the energy needed to remove a neutron from the nucleus of the calcium isotope 42 20 Ca. (b) Find the energy needed to remove a proton from this nucleus (c) Why are these energies different? M ( 41 20 Ca ) ( ) 40.962278 41.958622 u , M 42 u M ( n ) 1.008665 u , M ( p ) 1.007276u = = = 20 Ca (a) 40.962278u + 1.008665 u 41.970943u = u 0.012321u = → ∆M 41.970943u − 41.958622 = 11.48 MeV B = ( 0.012321 u ) × 931.49 MeV/u = (b) Removing a proton from 42 20 Ca leavs the potassium isotope 41 19 K (40.961827 u). A similar calculation give binding energy of 10.27 MeV for the missing proton (c) Difference is due electrostatic repulsion between protons Binding Energy Calculations M N - mass of the nucleus, M A - mass of the atom, me - mass of the electron, m p - mass of the proton, mn - mass of the neutron, Batom = M N c 2 + Zme c 2 − M Ac 2 = ∆mc 2 ≈ 0 (compared to Bnuclear ) B= Zm p c 2 + Nmn c 2 − M N c 2 = Zm p c 2 + Zme c 2 + Nmn c 2 − ( M N c 2 + Zme c 2 ) nuclear ZM H c 2 Bnuclear = ZM H c 2 + Nmn c 2 − M Ac 2 M Ac2 Liquid – Drop Model Semiempirical Mass Formula B =a1 A − a2 A2/3 − a3 Z ( Z − 1) A−1/3 − a4 ( A − 2 Z ) A−1 ± a5 A−1/2 2 23.7 Liquid – Drop Model 4 Evolume V = π R 3 a1 A → Evolume = 3 1/3 R = R0 A Reason why the binding energy per nucleon is approximately constant Liquid – Drop Model • Correction to first term − a2 A2/3 Esurface S = 4π R 2 → Esurface = R = R0 A1/3 • Implies fewer interactions for surface nucleus and thus smaller binding energy • Explains sharp decline in the binding energy per nucleon at low A values. Liquid – Drop Model • Correction to first term 2 3 ( Ze ) − a3 Z 2 A−1/3 Z 2 A−1/3 → Ecoulomb = Ecoulomb = 5 4πε 0 R 1/3 R = R0 A • Energy of repulsion decreases the binding energy (thus < 0) • Explains slow decline in the binding energy per nucleon at large A values. Semiempirical Mass Formula SEMF – Asymmetry Energy N − Z = A − Z − Z = A − 2Z ε energy incease 1 1 = − − N Z N Z ( ) ( ) 2 new neutron 2 2 ε ε 2 2 ∆E = − = − 2 N Z A Z ( ) ( ) →E 2 = ∆ = − − 2 E a A Z A−1 ( ) 8 8 assymetry 4 −1 εA ∆ = E ( number of new neutrons ) SEMF – Pairing Energy E pairing = ± a5 A−1/2 • The last term arises from the tendency of proton pairs and neutron pairs to occur. • Even-even nuclei are most stable and have higher binding energies than would otherwise expected • Such nuclei as 42 He, 12 6 C and 16 8 O appear as peaks on the empirical curve • Odd-Odd nuclei have both unpaired protons and neutrons and have relatively low B.E. Weizsäcker’s SEMF 23.7 23.7 More Binding Energy Calculations Example: The atomic mass of the zinc isotope 64 30 Zn is 63.929 u. Compare its binding energy with the prediction of SEMF Solution: The binding energy of 64 30 Zn is: Eb = ( 30 )(1.007825 u ) + ( 34 )(1.008665 u ) − 63.929 u × ( 931.49 MeV/u ) = 559.1 MeV Using SEMF: ( 0.75 MeV )( 30 ) Eb = (15.67 MeV )( 64 ) − (17.23 MeV )( 64 ) − 1/3 ( 64 ) 2/3 + 12 MeV ( 64 ) 1/2 554.1 MeV = Eb 559.1 Eb 554.1 = = 8.73 MeV or= = 8.66 MeV A 64 A 64 less than 0.1% difference 2 − ( 23.7 MeV )(16 ) 64 More Binding Energy Calculations Nuclear Exchange Force Nuclear Exchange Force Electrostatic Interaction (Classical Picture) Distribution of charge produces an electric field E , and the force felt by another charge q located in the field is the product qE. Any change in the charge distribution changes E , however, the information that change has occured does not appear instantaneously throughout the field, but it is propageted outward at the speed of light Electrostatic Interaction (Quantum Mechanical Picture) Every charge is continually emitting and absorbing photons, even when it is not moving. These photons are called virtual photons, meaning they are not directly observable. A charge can emmit a virtual photon of energy hf without changing its energy or recoiling. Energy and momentum conservation laws are not violated provided that photon exists for no longer than ∆t= / ∆E , hf , as required by uncertainty principle. where ∆E = R = c∆t = λ c c c = = = ∆E hf 2π f 2π Nuclear Exchange Force 1935 Hideki Yukawa Nuclear interaction is carried out by virtual particles - mesons Short Range - meson has a mass ∆E ≥ mc 2 c 1 fm = ∆E mc m ≈ 3.5 ×10−28 kg ≈ 380me ≈ 200 MeV/c 2 R = c∆t = Charge Independence - mesons carry + e, 0, - e charge Experimentaly π ± , π 0 are found in 1947 mπ ≈ 140 MeV/c 2 Nuclear Exchange Force Probability Density of the Exchange Mesons Virtual Meson Exists : ( ) 1.055 ×10−34 J×s −24 s ∆t = = = 5 × 10 2 2 2 −13 mc 140 MeV/c c 1.6 ×10 J/MeV ( )( )( ) Thus, a 10−20 second time-exposure "snapshot" of a nucleon would show a cloud consisting more than 10,000 mesons surrounding the nucleon! ( ) 2 2 ∂ Φ r , t ( ) 1 mc 2 ⇒ ∇ Φ r , t − = Φ r ,t) ( ) ( 2 2 ∂ c ∂t E → i , p 2 → − 2∇ 2 ∂t mc 2 = E 2 − ( pc ) 2 2 Probability Density of the Exchange Mesons 2 2 ∂ Φ r , t ( ) 1 mc ∇ 2Φ ( r , t ) − 2 = Φ r ( , t ) → For stationary solution → 2 c ∂t Ae − r / R mc 2 ∇ Φ ( r= ) Φ ( r ) → Φ ( r=) r 2 −2 r / R 2 A e Probability Density → Φ ( r ) = 2 r 2 → Probability Density of the Exchange Mesons • For r > 0.5 R curve agrees with experimental results. • Breaks down for r < 0.5 R • Measured meson density is much lower than figure would suggest • Reason - at r < 0.5 R quark composition of nuclei and mesons becomes important • Decrease in meson density is due to saturation of strong force - called assymtotic freedom of quarks. QCD – Asymptotic Freedom • For low r , strong force is diluted by gluon selfinteractions. • α S → 0 faster than 1 r • For r < 10−18 m, quarks move as almost free particles. • Asymtotic Freedom confirmed by electron deep scattering experimensts. QCD – Asymptotic Freedom Quark Confinement VQCD ( r ) = − 4α S 4α S + kr , FQCD ( r ) = −∇VQCD ( r ) = −k 2 3r 3r lim VQCD ( r ) → ∞, r →∞ lim FQCD ( r ) → const r →∞