* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Chemical Context of Life
Nuclear transmutation wikipedia , lookup
Chemical reaction wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Low-energy electron diffraction wikipedia , lookup
Marcus theory wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Halogen bond wikipedia , lookup
Electrochemistry wikipedia , lookup
Computational chemistry wikipedia , lookup
Livermorium wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Isotopic labeling wikipedia , lookup
Chemical element wikipedia , lookup
Biochemistry wikipedia , lookup
Periodic table wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Molecular orbital wikipedia , lookup
Hydrogen bond wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
History of chemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
Hydrogen atom wikipedia , lookup
Electronegativity wikipedia , lookup
Atomic orbital wikipedia , lookup
Bond valence method wikipedia , lookup
Bent's rule wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Molecular dynamics wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Extended periodic table wikipedia , lookup
Metallic bonding wikipedia , lookup
Atomic nucleus wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Electron configuration wikipedia , lookup
History of molecular theory wikipedia , lookup
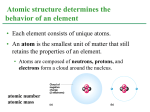
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 2 The Chemical Context of Life Lectures by Erin Barley Kathleen Fitzpatrick 1 © 2011 Pearson Education, Inc. Overview: A Chemical Connection to Biology • Biology is a multidisciplinary science • Living organisms are subject to basic laws of physics and chemistry • One example is the use of formic acid by ants to maintain “devil’s gardens,” stands of Duroia trees Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Figure 2.1 EXPERIMENT Cedrela sapling Insect barrier Duroia tree Inside, unprotected Devil’s garden Outside, protected Inside, protected Outside, unprotected RESULTS Dead leaf tissue (cm2) after one day Figure 2.2 16 12 8 4 0 Outside, Inside, Inside, Outside, unprotected protected unprotected protected Cedrela saplings, inside and outside devil’s gardens Concept 2.1: Matter consists of chemical elements in pure form and in combinations called compounds • Organisms are composed of matter 物質 • Matter is anything that takes up space and has mass* Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Elements and Compounds • Matter is made up of elements • An element 元素 is a substance that cannot be broken down to other substances by chemical reactions • A compound 化合物 is a substance consisting of two or more elements in a fixed ratio • A compound has characteristics different from those of its elements Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Fig. 2-3 The emergent properties of a compound. Sodium Chlorine Sodium chloride The Elements of Life • About 25 of the 92 elements are essential to life • Carbon, hydrogen, oxygen, and nitrogen make up 96% of living matter • Most of the remaining 4% consists of calcium, phosphorus, potassium, and sulfur • Trace elements are those required by an organism in minute quantities ex. Fe, I ect. Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Table 2.1 Fig. 2-4 (a) Nitrogen deficiency The effect of nitrogen deficiency in corn. In the controlled experiment, the taller plants on the left are growing in nitrogen-rich soil, and the shorter plants on the right in nitrogen-poor soil. (b) Iodine deficiency Goiter is an enlargement of the thyroid gland, resulting from a deficiency of the trace element iodine. It can probably be reversed by iodine supplements. Case Study: Evolution of Tolerance to Toxic Elements • Some elements can be toxic, for example, arsenic (As 砷) • Some species can become adapted to environments containing toxic elements – For example, some plant communities are adapted to serpentine , which contains chromium (Cr), nickel (Ni), and cobalt (Co) © 2011 Pearson Education, Inc. Figure 2.4 Concept 2.2: An element’s properties depend on the structure of its atoms • Each element consists of unique atoms 原子 • An atom is the smallest unit of matter that still retains the properties of an element Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Subatomic Particles • Atoms are composed of subatomic particles • Relevant subatomic particles include: – Neutrons 中子 (no electrical charge) – Protons 質子 (positive charge) – Electrons 電子 (negative charge) Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings • Neutrons and protons form the atomic nucleus 原子核 • Electrons form a cloud around the nucleus • Neutron mass and proton mass are almost identical and are measured in daltons (1.7 X 10-24 gram) © 2011 Pearson Education, Inc. Fig. 2-5 Cloud of negative charge (2 electrons) (a) Nucleus Electrons (b) Simplified models of a helium (He) atom. The helium nucleus consists of 2 neutrons (brown) and 2 protons (pink). Two electrons (yellow) exist outside the nucleus. Atomic Number and Atomic Mass • Atoms of the various elements differ in number of subatomic particles • An element’s atomic number 原子序 is the number of protons in its nucleus (ex. 2He) • An element’s mass number 質量數 is the sum of protons + neutrons in the nucleus (ex. 24He or 1123Na) • Atomic mass 原子量, the atom’s total mass, can be approximated by the mass number (ex. Na is 23 daltons (22.9898 daltons precisely)) Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Isotopes • All atoms of an element have the same number of protons but may differ in number of neutrons • Isotopes are two atoms of an element that differ in number of neutrons C isotopes All 3 have 6 protons - 612C, 98.93% Stable isotopes - 613C, 1.07% - 614C, even rare Radioactive isotopes (unstable isotopes) Atomic mass of C is 12.0107 Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Two isotopes of sodium. • Radioactive isotopes decay spontaneously, giving off particles and energy. – Number of protons changes, and transforms to a different element. Ex. Radioactive carbon → nitrogen. • Some applications of radioactive isotopes in biological research are: – Dating fossils – Tracing atoms through metabolic processes – Diagnosing medical disorders Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Figure 2.6a 10°C 15°C 20°C TECHNIQUE Compounds including 35°C Incubators radioactive tracer 45°C (bright blue) 20°C 10°C 15°C 50°C Human cells 1 Human cells are incubated with compounds used to make DNA. One compound is labeled with 3H. 2 Cells from each incubator are placed in tubes; their DNA is isolated; and unused labeled compounds are removed. 25°C 30°C 35°C 40°C 45°C 50°C 10° 15° 20° 25° 30° 35° 40° 45° 50° DNA (old and new) Figure 2.6b TECHNIQUE 3 The test tubes are placed in a scintillation counter. Figure 2.6c Counts per minute ( 1,000) RESULTS 30 20 Optimum temperature for DNA synthesis 10 0 10 20 30 40 50 Temperature (°C) Figure 2.7 A PET scan, a medical use for radioactive isotopes. PET, an acronym for positron-emission tomography, detects locations of intense chemical activity in the body. The bright yellow spot marks an elevated level of radioactively labelled glucose, which in turn indicated high metabolic activity, a hallmark of cancerous tissue. Cancerous throat tissue 核子試爆給的 生物線索 對瑞典斯德哥爾摩諾貝爾醫學研究所的弗瑞森(Jonas Frisen)來說,挫折是發明之母 。弗瑞森是一名神經科學家,專門研究腦組織的再生,他想知道人體各器官組織的年 齡,卻苦無方法。如果能為人體組織定年的話,他就能夠確定人類腦組織是否會部份 、或甚至完全再生,以及會多常再生。他說:「這個問題困擾著我。」一般用來標記 細胞然後觀察它們在動物體內生命週期的技術,由於使用到有毒化學藥劑,因此不適 用於人類,使得人類組織再生的問題,一直懸而未解。 然後弗瑞森得知1955年後出生的人,體內都帶有天然標記。自1955年到1963年制訂「 核子武器部份限定條約」為止,許多在地面上進行的核子武器試爆,釋放出大量的碳 14(14C)同位素到大氣中,這些14C很快飄浮擴散到全球,然後植物細胞攝取了14C ,動物吃植物,而以動、植物為食的人類,細胞也吸收了同位素,弗瑞森現在可以追 查這些14C的蹤跡。 透過測量DNA分子的14C含量,然後比對大氣層中14C含量,弗瑞森終於建立了一套能 夠回答細胞年齡疑問的試驗,2004年他將結果發表在《細胞》上,他發現人體中許多 部位都比整個身體年輕許多:30多歲的受試者,消化道組織的空腸細胞還不到16歲; 快40歲的受試者,其骨骼肌只有15歲。 地表上的核彈試爆導致大氣層 中14C激增900%,在人類組織 內留下了可以用來定年的微量 標記。 然而最讓科學家驚訝的是腦組織。弗瑞森檢查了小腦和大腦枕葉皮質的細胞,這兩個 組織的神經元,年紀都吻合或接近受試者的實際年齡,顯示這些部位在受試者出生時 就形成了,而通常不會進行更替。 這項發現是不是表示腦組織再生療法沒有希望,弗瑞森認為現在還言之過早。他承認 :「我不會這麼說,只是機會比有旺盛的再生現象小一點。」他迫不及待想繼續這項 研究,舉例來說,看看中風患者腦組織的神經元受損後能否再生,他也計畫研究心肌 和胰臟內製造胰島素的β細胞,這兩種組織的再生能力,一直引發激烈的爭議,也是 再生療法最感興趣的部位。弗瑞森希望其他研究人員也能利用這種技術,來研究他們 最感興趣的器官。 原子彈試爆時代殘留的14C在1965年後就快速減少,每11年減少一半,根據布赫茲的說 法,1990年後人類細胞殘留的同位素信號就很微弱。弗瑞森說,科學家可以為那段時 期儲存的組織樣本約略定年。現在布赫茲也將這種方法應用在法醫研究上。自己也出 生在該時期的布赫茲說:「嬰兒潮世代是個相當龐大的族群,這個技術肯定對我後半 期研究事業助益匪淺。」 【本文轉載自科學人2006年1月號】 The Energy Levels of Electrons • During a chemical reaction, their nuclei do no interact, but only electron involved. • Energy 能量 is the capacity to cause change • Potential energy 位能 is the energy that matter has because of its location or structure • The electrons of an atom differ in their amounts of potential energy • An electron’s state of potential energy is called its energy level, or electron shell 電子層 Fig. 2-8 (a) A ball bouncing down a flight of stairs provides an analogy for energy levels of electrons Third shell (highest energy level) Second shell (higher energy level) First shell (lowest energy level) (b) Atomic nucleus Energy absorbed Energy lost Electron Distribution and Chemical Properties • The chemical behavior of an atom is determined by the distribution of electrons in electron shells • The periodic table of the elements shows the electron distribution for each element Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Fig. 2-9 Electron-distribution diagrams for the first 18 elements in the periodic table. Hydrogen 1H Atomic mass First shell 2 He 4.00 Atomic number Helium 2He Element symbol Electrondistribution diagram Lithium 3Li Beryllium 4Be Boron 5B Carbon 6C Nitrogen 7N Oxygen 8O Fluorine 9F Neon 10Ne Silicon 14Si Phosphorus 15P Sulfur 16S Chlorine 17Cl Argon 18Ar Second shell Sodium Magnesium Aluminum 12Mg 11Na 13Al Third shell • Valence electrons 價電子 are those in the outermost shell, or valence shell 價層 • The chemical behavior of an atom is mostly determined by the valence electrons • Elements with a full valence shell are chemically inert (chemical unreactive); ex. He, Ne, Ar, etc. Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Electron Orbitals • An orbital 軌域 is the three-dimensional space where an electron is found 90% of the time • Each electron shell consists of a specific number of orbitals © 2011 Pearson Education, Inc. Figure 2.10 First shell Neon, with two filled Shells (10 electrons) Second shell (a) Electron distribution diagram First shell Second shell y x 1s orbital 2s orbital z Three 2p orbitals (b) Separate electron orbitals 1s, 2s, and 2p orbitals (c) Superimposed electron orbitals Concept 2.3: The formation and function of molecules depend on chemical bonding between atoms • Atoms with incomplete valence shells can share or transfer valence electrons with certain other atoms • These interactions usually result in atoms staying close together, held by attractions called chemical bonds 化學鍵 Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Covalent Bonds • A covalent bond 共價鍵 is the sharing of a pair of valence electrons by two atoms • In a covalent bond, the shared electrons count as part of each atom’s valence shell Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Fig. 2-11 Hydrogen atoms (2 H) Hydrogen molecule (H2) • A molecule consists of two or more atoms held together by covalent bonds • A single covalent bond, or single bond, is the sharing of one pair of valence electrons For example H:H (H–H) • A double covalent bond, or double bond, is the sharing of two pairs of valence electrons For example O::O (O=O) Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings • The notation used to represent atoms and bonding is called a structural formula 結 構式 – For example, H–H • This can be abbreviated further with a molecular formula 分子式 – For example, H2 Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Fig. 2-12 Name and Molecular Formula Two hydrogen atoms can form a single bone. Two oxygen atoms share two pairs of electrons to form a double bond. Two hydrogen atoms and one oxygen atom are joined by covalent bonds to produce a molecule of water. Four hydrogen atoms can satisfy the valence of one carbon atom, forming methane. ElectronLewis Dot Spacedistribution Structure and filling Model Diagram Structural Formula (a) Hydrogen (H2) (b) Oxygen (O2) (c) Water (H2O) (d) Methane (CH4) Animation: Covalent Bonds • Covalent bonds can form between atoms of the same element or atoms of different elements • A compound is a combination of two or more different elements • Bonding capacity is called the atom’s valence 價數 Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings • Electronegativity 陰電性 is an atom’s attraction for the electrons in a covalent bond • The more electronegative an atom, the more strongly it pulls shared electrons toward itself Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings • In a nonpolar covalent bond 非極性共價 鍵, the atoms share the electron equally, ex. H2, O2, CH4. • In a polar covalent bond 極性共價鍵, one atom is more electronegative, and the atoms do not share the electron equally, ex. H2O • Unequal sharing of electrons causes a partial positive or negative charge for each atom or molecule Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Fig. 2-13 Because O is more electronegative than H, shared electrons are pulled more toward O. – This results in a partial negative charge on the oxygen and a partial positive charge on the hydrogen. O + H H H2O + Ionic Bonds • Atoms sometimes strip electrons from their bonding partners • An example is the transfer of an electron from sodium to chlorine • After the transfer of an electron, both atoms have charges • A charged atom (or molecule) is called an ion離子 Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Fig. 2-14 Electron transfer and ionic bonding The lone valence electron of a sodium atom is transferred to join the 7 valence electrons of a chlorine atom. Each resulting ion has a completed valence shell. An ionic bond can form between the oppositely charged ions. Na Cl Na Cl Na Sodium atom Cl Chlorine atom Na+ Sodium ion (a cation) Cl– Chloride ion (an anion) Sodium chloride (NaCl) • A cation 陽離子 is a positively charged ion • An anion 陰離子 is a negatively charged ion • An ionic bond 離子鍵 is an attraction between an anion and a cation Animation: Ionic Bonds Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings • Compounds formed by ionic bonds are called ionic compounds, or salts • Salts, such as sodium chloride (table salt), are often found in nature as crystals Na+ Cl– Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Weak Chemical Bonds • Most of the strongest bonds in organisms are covalent bonds that form a cell’s molecules • Weak chemical bonds, such as ionic bonds and hydrogen bonds, are also important • Weak chemical bonds reinforce shapes of large molecules and help molecules adhere to each other Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Hydrogen Bonds • A hydrogen bond forms when a hydrogen atom covalently bonded to one electronegative atom is also attracted to another electronegative atom • In living cells, the electronegative partners are usually oxygen or nitrogen atoms Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Fig. 2-16 + Water (H2O) + Hydrogen bond Ammonia (NH3) + + + Van der Waals Interactions • If electrons are distributed asymmetrically in molecules or atoms, they can result in “hot spots” of positive or negative charge • Van der Waals interactions are attractions between molecules that are close together as a result of these charges Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Figure 2.UN01 • Collectively, such interactions can be strong, as between molecules of a gecko’s toe hairs and a wall surface Molecular Shape and Function • A molecule’s shape is usually very important to its function • A molecule’s shape is determined by the positions of its atoms’ valence orbitals • In a covalent bond, the s and p orbitals may hybridize, creating specific molecular shapes © 2011 Pearson Education, Inc. Figure 2.17 s orbital Four hybrid orbitals z Three p orbitals x y Tetrahedron (a) Hybridization of orbitals Space-Filling Model Ball-and-Stick Model Hybrid-Orbital Model (with ball-and-stick model superimposed) Unbonded Electron pair 104.5o Water (H2O) Methane (CH4) (b) Molecular-shape models • Biological molecules recognize and interact with each other with a specificity based on molecular shape • Molecules with similar shapes can have similar biological effects © 2011 Pearson Education, Inc. Figure 2.18 A molecular mimic Carbon Hydrogen Natural endorphin Nitrogen Sulfur Oxygen Morphine Morphine affects pain perception and emotional state by mimicking the brain’s natural endorphins 腦內啡 (a) Structures of endorphin and morphine Natural endorphin Brain cell Morphine Endorphin receptors (b) Binding to endorphin receptors Concept 2.4: Chemical reactions make and break chemical bonds • Chemical reactions are the making and breaking of chemical bonds • The starting molecules of a chemical reaction are called reactants • The final molecules of a chemical reaction are called products Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Fig. 2-UN2 2 H2 O2 Reactants 2 H2 O Reaction Products • Photosynthesis is an important chemical reaction • Sunlight powers the conversion of carbon dioxide and water to glucose and oxygen 6 CO2 + 6 H20 → C6H12O6 + 6 O2 Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Fig. 2-19 • Some chemical reactions go to completion: all reactants are converted to products • All chemical reactions are reversible: products of the forward reaction become reactants for the reverse reaction • Chemical equilibrium is reached when the forward and reverse reaction rates are equal Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings You should now be able to: 1. Identify the four major elements 2. Distinguish between the following pairs of terms: neutron and proton, atomic number and mass number, atomic weight and mass number 3. Distinguish between and discuss the biological importance of the following: nonpolar covalent bonds, polar covalent bonds, ionic bonds, hydrogen bonds, and van der Waals interactions Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings