* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Antibiotic distribution into the CNS
Discovery and development of tubulin inhibitors wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Discovery and development of cephalosporins wikipedia , lookup
Drug discovery wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Drug design wikipedia , lookup
Psychopharmacology wikipedia , lookup
Drug interaction wikipedia , lookup
Neuropharmacology wikipedia , lookup
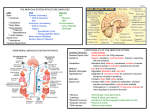
Antibiotics and the CNS M Armstrong Antibiotic penetration into the CNS • Pharmacokinetic data concerning the entry of drugs into the CSF is incomplete -Physical barrier: blood-CSF barrier (or blood brain) -Enzymatic blood-brain barrier Different CNS compartments Distribution of drugs within the CNS is not uniform (think CSF, extracellular and intracellular spaces within the brain and spinal cord) -most drugs achieve higher concentrations in the lumbar CSF vs ventricular CSF However no diffusional barrier exists between the interstitial space of the nervous system and the CSF In theory there should be good correlation between CSF levels and interstitial levels But…CSF is in constant flux from extracellular fluid (1/3rd of CSF originates from the extracellular space of neurons) to the “CSF space” and then into the venous system The majority of anti-infections that have reached steady state concentrations in the CSF, have levels substantially lower than the corresponding plasma concentration -AUCcsf/AUCs or the steady state concentration of CSF drug concentration to serum drug concentration is the most accurate parameter characterizing drug penetration into the CSF Additional host factors such as meningeal inflammation* and age as well as physicochemical properties of drugs are also important *drug concentrations measured in the absence of meningeal inflammation represent the minimal concentrations that can be encountered in early or resolving CNS infection Physicochemical properties Molecular size: The smaller the better for CNS penetration Lipophilicity: The more lipophilic usually the better- although very lipophilic molecules are often highly protein bound or bind to lipid membranes. Diffusion can also be pH dependent Plasma protein binding: Generally increased binding results in less penetration into the CNS Physicochemical properties Active transport systems: Variable effect on different drugs but usually active transport is out of the CNS (P-glycoprotein, Oat3, PEPT2) Upregulation of active transport system can occur Metabolism within the CNS: Certain drugs are more susceptible then others e.g. the choroid plexus are able to metabolize phenobarbital, dexamethasone and cephalothin Meningeal inflammation Results in blood brain barrier becoming “leaky” as tight junctions break down CSF outflow resistance also increases, leading to moderate CSF production and absorption rate Active transport mechanisms can also be inhibited Beta-Lactams 400 Da, 0-95% plasma protein binding In absence of meningeal inflammation penetration is usually poor (AUCcsf/AUCs around 0.15) High doses can be tolerated, which can get over the issue of poor penetration *but can decrease the seizure threshold Potential issues with PK differences in BL-BLI combinations in CNS infection Aminoglycosides 400 Da, hydrophilic, low plasma protein binding CSF penetration considered poor, however is not lower then that of beta-lactams and other hydrophilic antibiotics of a similar mass Therapeutic index is low- ? Role of intrathecal administration Fluroquinolones 300 Da, Moderately lipophilic, low binding to plasma proteins (20-40%) Penetration into CSF in non-inflamed meninges much higher than for beta-lactams Utility in treating gram negative aerobic bacilli and TB Not feasible in treating other pathogens such as Streptococcus pneumonia because activity to low and unable to increase dose significantly because of relative high CNS toxicity Chloramphenicol Small molecule, active against a wide range of bacteria Readily enters the CSF Previous extensive use to treat meningitis Lawyers’ limit its use (in industrialized countries) Macrolides Highly lipophilic, 750 Da, high affinity for P-gp Limited CNS penetration Role is limited to CNS infections 2nd to Mycoplasma sp. And Legionella pneumophila encephalitis Tetracyclines and Tigecycline Doxycycline is lipophilic, with protein binding over 80% CNS penetration 0.2 in absence and presence of inflammation of meninges (usually dose 400mg per day) Not inferior to Ceftriaxone in treating neuroborreliosis. Can be used for neurobrucellosis and even neurosyphilis Tigecycline – CNS penetration 0.1 in uninflamed meninges Fosfomycin 138 Da, hydrophilic, not protein bound Enters CNS more readily then beta-lactams Use in Europe of intravenous fosfomycin in treating some CNS infection by multi-resistant bacteria Oxazolidinones Linezolid is amphiphilic and has a CNS penetration ration of close to 1 Although primary bacteriostatic, potential utility in gram positive CNS infection by resistant organisms Metronidazole and Clindamycin Metronidazole: small, lipophilic molecule -Good penetration into CSF and absesses Clindamycin: 425 Da, with poor CNS penetration, but can be used in high doses potentially Rifamycins Lipophilic, > 800 Da, protein binding around 80% Moderate CNS penetration, with CNS concentrations often independent of the state of the blood-CSF barrier Side effects of limit use for CNS infection Sulfonamides and Trimethoprim Small lipophilic molecules Good CNS penetration, higher then that of betalactams Not so dependent of meningeal inflammation Use in certain infections- Listeria sp, Nocardia sp, Stenotropomonas, toxoplasmosis Bone marrow toxicity may complicate use Glycopeptides Hydrophilic antibiotics with high mass (over 1400 Da) Protein binding <50% for vancomycin, >90% for teicoplanin Patients with inflamed meninges usually optain good therapy levels in CSF Concurrent dexamethasone may reduce CSF levels (? Use ceftriaxone and rifampicin instead of vanc in empiric meningitis therapy) Toxicity can limit dose increases Daptomycin and fusidic acid While daptomycin only has 6% CNS penetration, has previously been used to treat MRSA meningitis with success Fusidic acid has poor CNS penetration Colistin Poor CNS penetration Used in carbapenem resistant Acinetobacter sp CNS infection Can use intra-thecally Anti-tuberculosis drugs Pyrazinamide and isoniazid are moderately lipophilic and small molecules and have good CNS penetration Other first line agents have poor penetration Moxifloxacin is a useful alternative Anti-herpes virus drugs Moderate for oral administration CSF penetration of ganciclovir 0.155 in non-inflamed meninges (similar to acyclovir) Antiretrovirals HIV encephalitis does not have a significant effect on the blood-CSF/blood brain barrier Wide variation in compounds studied, but generally PI’s have poorer CNS penetration (often ligands of P-gp) CNS penetration-effectiveness (CPE) index -zidovudine (0.75), abacavir (0.35), delavirdine, nevirapine, indinavir, lopinavir Antifungals Amphotericin (all formulations), has poor CNS penetration -although still often used for CNS disease -intrathecal administration possible 5-Flucytosine has good CNS penetration (0.75) Echinocandins have poor CNS penetration Azoles- voriconazole (0.46) and fluconazole are the best Antiparasitics Pyrimethamine (cerebral toxoplasmosis), has CSF levels about 10-25% of serum levels Atorvaquone has no studies but has had some clinical success Albendazole has good CNS penetration, compared to Praziquantel Reference Nau et al. Penetration of Drugs through the Blood-Cerebrospinal Fluid/Blood Brain Barrier for Treatment of Central Nervous System Infections. Clin. Microbiol. Rev. 2010, 23(4):858-883.