* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 6-Respiratory_chain
Microbial metabolism wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Citric acid cycle wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Light-dependent reactions wikipedia , lookup
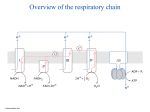
The respiratory chain © Michael Palmer 2016 1 Overview of the respiratory chain © Michael Palmer 2016 2 Functional stages in the respiratory chain 1. H2 is abstracted from NADH+H+ and from FADH2 2. The electrons obtained with the hydrogen are passed down a cascade of carrier molecules located in complexes I–IV, then transferred to O2 3. Powered by electron transport, complexes I, III, and IV expel protons across the inner mitochondrial membrane 4. The expelled protons reenter the mitochondrion through ATP synthase, driving ATP synthesis © Michael Palmer 2016 3 Uncoupling proteins dissipate the proton gradient © Michael Palmer 2016 4 The uncoupling action of dinitrophenol © Michael Palmer 2016 5 The Racker experiment: bacteriorhodopsin can drive ATP synthase © Michael Palmer 2016 6 Molecules in the electron transport chain © Michael Palmer 2016 7 Iron-containing redox cofactors © Michael Palmer 2016 8 Flavin-containing redox cofactors © Michael Palmer 2016 9 The respiratory chain produces reactive oxygen species as byproducts © Michael Palmer 2016 10 Redox reactions can be compartmentalized to produce a measurable voltage © Michael Palmer 2016 11 Energetics of electron transport • Each electron transfer step along the chain is a redox reaction: the first cofactor is oxidized and the second one is reduced • In a redox reaction, electrons flow spontaneously if the reduction potential increases in the forward direction (ΔE > 0) • Redox reactions, like other reactions, proceed spontaneously if their free energy is negative (ΔG < 0) • Question: How is the reduction potential of a redox reaction related to its free energy? © Michael Palmer 2016 12 The redox potential (ΔE) is proportional to the free energy (ΔG) © Michael Palmer 2016 13 Redox potentials and free energies in the respiratory chain © Michael Palmer 2016 14 The first two redox steps in complex I © Michael Palmer 2016 15 The reduction of coenzyme Q involves protons and electrons © Michael Palmer 2016 16 The Q cycle (criminally simplified) © Michael Palmer 2016 17 Reduction of oxygen by cytochrome C oxidase (complex IV) © Michael Palmer 2016 18 How is electron transport linked to proton pumping? • Some redox steps in the ETC are coupled to proton binding and dissociation, which may occur at opposite sides of the membrane. Example: Coenzyme Q cycle at complex III • Redox steps that do not involve hydrogen directly need a different mechanism in order to contribute to proton pumping. Example: Sequence of iron-sulfur clusters and hemes in complex IV © Michael Palmer 2016 19 Linking electron movement to proton pumping: A conceptual model © Michael Palmer 2016 20 Proton pumping creates both a concentration gradient and a membrane potential © Michael Palmer 2016 21 Structure of ATP synthase © Michael Palmer 2016 22 The binding-change model of ATP synthase catalysis © Michael Palmer 2016 23 How does proton flux drive ATP synthase? © Michael Palmer 2016 24 Proton flux causes c chains to rotate within the F0 disk © Michael Palmer 2016 25 A hypothetical malate-oxaloacetate shuttle © Michael Palmer 2016 26 The malate-aspartate shuttle © Michael Palmer 2016 27 The glycerophosphate shuttle © Michael Palmer 2016 28 The two mitochondrial isocitrate dehydrogenases © Michael Palmer 2016 29 Nicotinamide nucleotide transhydrogenase couples hydrogen transfer with proton transport © Michael Palmer 2016 30 At rest, transhydrogenase and the two isocitrate dehydrogenases form a futile cycle © Michael Palmer 2016 31 When ATP demand is high, transhydrogenase turns into an auxiliary proton pump © Michael Palmer 2016 32 Theoretical ATP per molecule of glucose completely oxidized Quantity Intrinsic value Accrued hydrogen © Michael Palmer 2016 Per glucose 10 NADH, 2 FADH2 Protons ejected 10 per NADH, 6 per FADH2 112 Proton-powered ATP synthase revolutions 10 protons per revolution 11.2 ATP from ATP synthase 3 per revolution 33.6 ATP from glycolysis 2 GTP from TCA cycle 2 Total 37.6 33 Processes other than ATP synthesis that are powered by the proton gradient • Nicotinamide nucleotide transhydrogenase • Uncoupling proteins; proton leak • Secondary active transport: ◦ ATP4−/ADP3− antiport ◦ phosphate/H+ symport ◦ amino acid/H+ symport ◦ pyruvate/H+ symport © Michael Palmer 2016 34