* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Transition Metales
Hydroformylation wikipedia , lookup
Jahn–Teller effect wikipedia , lookup
Bond valence method wikipedia , lookup
Metal carbonyl wikipedia , lookup
Cluster chemistry wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Oxidation state wikipedia , lookup
Spin crossover wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
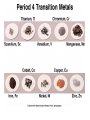
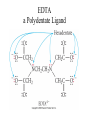
Transition Metals and Coordination Chemistry Dr.Monther F.Salem d-block elements transition metals d-block elements are: • all metals • all have partially filled d subshells • exhibit horizontal & vertical similarities • alloys & compounds are important components of materials in modern world • most first-row transition metals are essential for life Transition Metals • the properties of the transition metals are similar to each other – and very different to the properties of the main group metals – high melting points, high densities, moderate to very hard, and very good electrical conductors • in general, the transition metals have two valence electrons – we are filling the d orbitals in the shell below the valence – Group 1B and some others have 1 valence electron due to “promotion” of an electron into the d sublevel to fill it – form ions by losing the ns electrons first, then the (n – 1)d Transition Metals • Some differences – Melting point, Tungsten melts 3400°, while mercury -39°C – Some soft, like sodium that can be cut with a butter knife – Reactivity • Some spontaneously react with oxygen like iron, which flakes off • Others react with oxygen to make a colorless tight fitting oxide, such as chromium, thus protecting the surface • Some metals are inert to oxygen such as gold, silver and platinum Transition Metals Ionic compound formation – More than one oxidation state is often observed – Cations, often are complexes – Most compounds are colored, since complexes absorb visible light – Many compounds are paramagnetic Transition elements on the periodic table Dr.Monther F.Salem Aqueous solutions containing metal ions Co+2 Mn+2 Cr+3 Dr.Monther F.Salem Fe+3 Ni+2 Dr.Monther F.Salem Dr.Monther F.Salem Colors of Representative Compounds of the Period 4 Transition Metals b a d c f e a = Scandium oxide b = Titanium(IV) oxide c = Vanadyl sulfate dihydrate d = Sodium chromate e = Manganese(II) chloride tetrahydrate h g j i f = Potassium ferricyanide g = Cobalt(II) chloride hexahydrate h = Nickel(II) nitrate hexahydrate i = Copper(II) sulfate pentahydrate j = Zinc sulfate heptahydrate Orbital Occupancy of the Period 4 Metals–I Element Sc Partial Orbital Diagram 4s 3d 4p Unpaired Electrons 1 Ti 2 V 3 Cr 6 Mn 5 Orbital Occupancy of the Period 4 Metals–II Element Fe Partial Orbital Diagram 4s 3d 4p Unpaired Electrons 4 Co 3 Ni 2 Cu 1 Zn 0 Reactivity: Size of neutral atoms of d-block elements gradually decreases left to right across a row. Due to increase in Zeff with increasing atomic # Atomic radius increases going down a column. Oxidation States The common oxidation states for each element include +2, +3, or both. The first-row transition metals. The most stable oxidation numbers are shown in color. The zero oxidation state is encountered in some compounds, such as Ni(CO)4 and Fe(CO)5. Titanium bicycle Dr.Monther F.Salem General Trends among Transition Metals Dr.Monther F.Salem First-row Transition Metals Scandium – Rare element most always +3 oxidation state, ie ScCl3, Sc2O3 – Chemistry of scandium resembles the lanthanides First-row Transition Metals Titanium – Found in the earths crust (0.6%) – Low density and high strength – Fairly inert, and is used in pipes – TiO2 is a very common white pigment – Common oxidation state is +4 First-row Transition Metals Vanadium – Found in the earth’s crust about 0.02% – Common oxidation state is +5 – Since vanadium contains d electrons solutions are colored First-row Transition Metals Chromium – Rare, but important industrial chemical – Chromium oxide is colorless, tuff, and holds to the metal strongly, almost in visible – Chromium compounds in solution are also colored since they contain d electrons – Common oxidation states are +2, +3 and +6 First-row Transition Metals • Iron – Is the most abundant heavy metal (4.7%) in earth’s crust, – Common oxidation states +2 and +3 – Iron solutions are colored since they contain d electrons First-row Transition Metals • Cobalt – Relatively rare – Hard bluish-white metal – Common oxidation states are +2 and +3 First-row Transition Metals • Nickel – Most always the +2 oxidation state – Sometimes +3 oxidation state – Emerald green colored solutions First-row Transition Metals • Copper – Quite common, as sulfides, chlorides and carbonates – Great electrical conductor second only to silver – Widely used in plumbing – Not highly reactive will not reduce H+ – Slowly oxides in air, producing a green oxide – Common oxidation state +2, +1 is also known – Aqueous solution are bright Royal blue – Quite toxic, used to kill bacteria – Paint often contains copper so algae do not grow on the paint First-row Transition Metals Zinc – Quite common in earths crust, usually as ZnS – Great reducing agent, quite reactive – Oxidation state of +2 – Used to galvanize steel Coordination Compounds • when a complex ion combines with counterions to make a neutral compound it is called a coordination compound • the primary valence is the oxidation number of the metal • the secondary valence is the number of ligands bonded to the metal – coordination number • coordination number range from 2 to 12, with the most common being 6 and 4 CoCl36H2O = [Co(H2O)6]Cl3 Coordination Compound Complex Ion Formation • complex ion formation is a type of Lewis acid-base reaction • a bond that forms when the pair of electrons is donated by one atom is called a coordinate covalent bond Ligands • Atoms attached to a transition metal via coordinate covalent bonds • They are Lewis bases, since they donate a pair of electrons to the transition metal. • Ligands are classified relative to how many attachments to the metal – Monodentate forms one bond to a transition metal – Lignads forming more than bond are called chelating ligands, or chelates Ligands • Ligands are classified relative to how many attachments to the metal – Bidentate, a chelating agent, forms two bonds, examples: • Oxalate • Ethylenediamine – Polydentate forms more than two bonds. • Diethylenetriamine • Ethylenediaminetetraacetic acid Ligands • EDTA is used to remove lead from animals • More complicated ligands are found in biological compounds • EDTA is used as a preservative to tie up substances that could catalyze decomposition of food products Ligands with Extra Teeth • some ligands can form more than one coordinate covalent bond with the metal atom – lone pairs on different atoms that are separate enough so that both can reach the metal • chelate is a complex ion containing a multidentate ligand – ligand is called the chelating agent EDTA a Polydentate Ligand Complex Ions with Polydentate Ligands Geometries in Complex Ions Coordination Compounds • The number of coordinate covalent bonds formed by the metal ion and the ligands. • Geometrical Common Shape – Ligands = 2, then linear – Ligands = 4, then tetrahedral, or square planar – Ligands = 6, then octahedral and prismatic (rare) Ligand arrangements for coordination numbers 2, 4, and 6 Dr.Monther F.Salem