* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download General Chemistry: An Integrated Approach
James Franck wikipedia , lookup
Elementary particle wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Particle in a box wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Double-slit experiment wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Atomic orbital wikipedia , lookup
Matter wave wikipedia , lookup
Hydrogen atom wikipedia , lookup
Tight binding wikipedia , lookup
Electron configuration wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
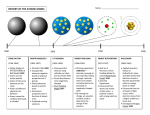
Chapter Seven Atomic Structure Views on Atomic Structure Classical View – electrons and properties of electrons Experiments with Light – Quantum Theory Quantum View – behavior of electrons in atoms EOS Chapter 7: Atomic Structure 2 Cathode Rays Cathode rays are the carriers of electric current from cathode to anode inside a vacuumed tube Emit perpendicular to the cathode surface when electricity is passed through an evacuated tube ... ... and travel in straight lines EOS Chapter 7: Atomic Structure 3 Cathode Rays Cause glass and other materials to fluoresce Deflect in a magnetic field similarly to negatively charged particles EOS Chapter 7: Atomic Structure 4 J. J. Thomson’s Experiment Devised an experiment to find the ratio of the cathode ray particle’s mass (me) to the charge (e) me /e = –5.686 × 10–12 kg C–1 EOS Chapter 7: Atomic Structure 5 The Electron Millikan measured the charge on an electron - the famous “oil drop” experiment. EOS Chapter 7: Atomic Structure 6 Determined Electron Values Robert Millikan then determined a value for the charge e = –1.602 × 10–19 C From m/e and the charge, the mass of an electron was determined to be m = 9.109 × 10–31 kg/electron EOS Chapter 7: Atomic Structure 7 J. J. Thomson – Atomic Model Thomson proposed an atom with a positively charged sphere containing equally spaced electrons inside Proposed for a hydrogen atom that there was one electron at the exact center of the sphere Proposed for a helium atom that two electrons existed along a straight line through the center, with each electron being halfway between the center and the outer surface of the sphere EOS Chapter 7: Atomic Structure 8 Raisin Pudding Model EOS Chapter 7: Atomic Structure 9 Rutherford’s Model Ernest Rutherford characterized alpha particles through an experiment and discovered the positive charge of an atom is concentrated in the center of an atom, the nucleus EOS Chapter 7: Atomic Structure 10 Rutherford’s Interpretation EOS Chapter 7: Atomic Structure 11 Protons and Neutrons From Rutherford’s experiments, he was able to determine the amount of positive nuclear charge The positive charge was carried by particles called protons Scientists introduced the atomic number, which represents the number of protons in the nucleus of an atom James Chadwick discovered neutrons in the nucleus, which have nearly the same mass as protons and no charge Chapter 7: Atomic Structure EOS 12 Mass Spectrometry A mass spectrometer is a device that separates positive gaseous ions according to their mass-to-charge ratios A record of the separation of ions is called a mass spectrum EOS Chapter 7: Atomic Structure 13 Mass Spectrometer If a stream of positive ions having equal velocities is brought into a magnetic field, the lightest ions are deflected the most, making a tighter circle EOS Chapter 7: Atomic Structure 14 The Atom – Where Were We? • The atom has gone through some changes, where are we now? • 1. Democritus/Dalton = small, spheres. • 2. Thomson = plum pudding model. • 3. Rutherford = planetary model. • The model is incomplete – it didn’t really explain where electrons were outside the nucleus. New Model of the Atom • In the early 20th century, a new model of the atom was proposed. • This new model evolved as a result of investigations into the absorption and emission of light by matter. • That’s where our story begins now…. Properties of Light • Light has been known for years to behave as a wave. • However, it was discovered that light also has particle-like characteristics. Wave Description of Light • What is light? • Light is a form of electromagnetic radiation. • Electromagnetic radiation = a form of energy that exhibits wavelike behavior as it travels through space. Examples of Electromagnetic Radiation • Electromagnetic radiation include X-rays, UV rays, infrared rays, and visible light. • What do you think is strong radiation? Weak radiation? • Together, all the forms of electromagnetic radiation form the electromagnetic spectrum. EM Radiation Spectrum Visible Light Parts of a Wave Parts of a Wave (Definitions) • c = the speed of a wave which is ALWAYS 3.0 x 108 m/s l = wavelength of a wave (usually in units of nm, m, or cm) • This l referred to as lambda. • The wavelength is the distance between corresponding points on adjacent waves. Parts of a Wave Parts of a Wave (Definitions) n = refers to the frequency of a wave n is known as “nu”. • The frequency of a wave is defined as the # of waves that pass a given point in a specific time. • Frequency has units of “waves/s”, “1/s”, “s-1”, or “Hz = Hertz”. Parts of a Wave (Definitions) • Amplitude (a) = is the height or brightness (loudness) of a wave. • The amplitude of a wave is measured in units of m, nm, or cm. Calculations • The overall formula we will use is this: • c = ln HINTS • HINT #1: Use a triangle. c is up top. • HINT #2: 1 nm = 1 x 10-9 m • HINT #3: frequency has MANY units. Remember that! • HINT #4: c is ALWAYS 3.0 x 108 m/s • HINT # 5: wavelength needs to be in “m”. Examples • I have a wave that has a wavelength of 1.20 x 10-9 m. What is the frequency of this wave? • I have a wave that has a frequency of 5.2 x 1016 Hz. What is the wavelength of this wave? Examples (Cont.) • I have a wave that has a wavelength of 750 nm. What is the frequency? • What part of the EM spectrum is this wave located? • I have a wave with a frequency of 3.5 x 1017 s-1. What is the wavelength? • What part of the EM spectrum is this wave located? POP Quiz!!!! • Given: Wavelength of 500 nm; Find = frequency of the wave. • What part of the EM is this wave? • Given: Frequency of 1.88 x 1015 Hz; Find = wavelength of the wave in BOTH m and nm. • What part of the EM is this wave? The Photoelectric Effect • Photoelectric effect uses the frequencies of various radiation. • Photoelectric effect = the emission of electrons from a metal when light shines on the metal. • What frequency of light do YOU think would eject or emit electrons from a metal? The Photoelectric Effect • Certain types of light, such as RED and ORANGE, do NOT hit metals and get electrons ejected. • However, BLUE, INDIGO, and VIOLET will. • Examples include the solar calculator, papertowel dryers, electronic doors. Light – Wave AND Particle? • The photoelectric effect helped to explain that particles of light (or photons) were what ejected these electrons. • This suggested the light not only had wavelike properties, but also particle-like properties. Particle Properties of Light • German physicist Max Planck suggested that objects emit energy in small packets of photons, referred to as quanta. • A quantum of energy is the minimum quantity of energy that can be lost or gained by an atom. • E = hn E = hn • E = energy, in Joules, of a quantum of radiation. n = frequency of radiation, in Hz. • h = Planck’s constant = 6.6261 x 10-34 J s. • This equation allows us to calculate the energy of EM radiation. Examples • Infrared light has a frequency of 7.29 x 1014 Hz. What is the energy associated with this light? • The energy of a photon of light is found to be 4.55 x 10-19 J. What is the frequency of this photon? Toughie! • A wave of violet light has a wavelength of about 400 nm. • What is (a) the frequency of this light? • And (b) the energy associated with this light, in Joules? Another tough one… • Calculate the wavelength emitted in the far UV that corresponds to an energy of 1609 kJ/mol photons. Chapter 7: Atomic Structure 39 The Hydrogen-Atom Line Emission Spectrum • Elements, such as hydrogen, produce various “lines” or emissions of light when current is passed through them. • Ground state = the lowest energy state if an atom. • The ground state is where atoms would like to be. Emission Spectra • Excited state(s) = state(s) in which an atom has a higher potential energy than the ground state. • When electrons get “zapped” by photons of energy, they go from the ground state to an excited state. • When an electron gets “tired” in the excited state, it emits energy, usually some color of visible light. Emission Spectra • When elements emit light into specific frequencies of visible light, it is referred to as a line-emission spectrum. • Continuous spectrum = the emission of a continuous range of frequencies of EM radiation. • Elements don’t follow a continuous spectrum – they emit specific frequencies of light. Bohr Model of the H-Atom • Niels Bohr, a Danish physicist, proposed a hydrogen-atom model that linked the electron to the photon. • He suggested the 1 electron in an H-atom is in an “energy level” closest to the nucleus. • He stated electrons move in “orbits” of energy, and the lowest energy level is referred to as ground-state energy level. Bohr Model of the H Atom • Bohr stated that the electron absorbed a fixed amount of energy (a photon) to go from the ground state to a higher energy state. • The electron absorbed or emitted a fixed amount of energy as it moved from various energy levels. Bohr Model of the H Atom • Ephoton = E2-E1 • This Bohr model of the atom seemed to work – but ONLY for the H atom. • WHY?!?!?!? • H only has 1 electron……. Wave Motion Caused by a displacement in a medium Characterized by height of crest (or depth of trough) EOS Chapter 7: Atomic Structure 46 The Wave Nature of Light Electromagnetic waves originate from the movement of electric charges The movement produces fluctuations in electric and magnetic fields Chapter 7: Atomic Structure 47 Characterizing Waves Electromagnetic radiation is characterized by its wavelength, frequency, and amplitude Wavelength (l) is the distance between any two identical points in consecutive cycles EOS Chapter 7: Atomic Structure 48 Characterizing Waves Frequency of a wave is the number of cycles of the wave that pass through a point in a unit of time Amplitude of a wave is its height: the distance from a line of no disturbance through the center of the wave peak EOS Chapter 7: Atomic Structure 49 The Electromagnetic Spectrum The electromagnetic spectrum is largely invisible to the eye EOS Chapter 7: Atomic Structure 50 The Electromagnetic Spectrum • We can feel some radiation through other senses (infrared) • Sunburned skin is a sign of too much ultraviolet radiation • Materials vary in their ability to absorb or transmit different wavelengths – Our bodies absorb visible light, but transmit most X rays – Window glass transmits visible light, but absorbs ultraviolet radiation EOS Chapter 7: Atomic Structure 51 Continuous Spectra White light passed through a prism produces a spectrum – colors in continuous form. EOS Chapter 7: Atomic Structure 52 The Continuous Spectrum l ~ 650 nm l ~ 575 nm l ~ 500 nm l ~ 480 nm l ~ 450 nm The different colors of light correspond to different wavelengths and frequencies EOS Chapter 7: Atomic Structure 53 Line Spectra Light passed through a prism from an element produces a discontinuous spectrum of specific colors EOS Chapter 7: Atomic Structure 54 Line Spectra The pattern of lines emitted by excited atoms of an element is unique = atomic emission spectrum EOS Chapter 7: Atomic Structure 55 Quantum Theory – Black Body Radiation Planck proposed that the vibrating atoms in a heated solid could absorb or emit electromagnetic energy only in discrete amounts The smallest amount of energy, a quantum, is given by: E = hv where h is Planck’s constant: = 6.626 × 10–34 J s Planck’s quantum hypothesis states that energy can be absorbed or emitted only as a quantum or as whole multiples of a quantum EOS Chapter 7: Atomic Structure 56 Quantum View of Atomic Structure Bohr’s Hydrogen Atom • Niels Bohr followed Planck’s and Einstein’s lead by proposing that electron energy (En) was quantized. • The electron in an atom could have only certain allowed values of energy (just as energy itself is quantized). • Each specified energy value is called an energy level of the atom: En = –B/n2 – n is an integer, and B is a constant (2.179 × 10–18 J) – The negative sign represents force of attraction. • The energy is zero when the electron is located infinitely far from the nucleus. Example 7.5 Calculate the energy of an electron in the second energy level of a hydrogen atom. The Bohr Model of Hydrogen When excited, the electron is in a higher energy level. Excitation: The atom absorbs energy that is exactly equal to the difference between two energy levels. Each circle represents an allowed energy level for the electron. The electron may be thought of as orbiting at a fixed distance from the nucleus. Emission: The atom gives off energy—as a photon. Upon emission, the electron drops to a lower energy level. Line Spectra Arise Because … Transition from n = 3 to n = 2. Transition from n = 4 to n = 2. • … each electronic energy level in an atom is quantized. • Since the levels are quantized, changes between levels must also be quantized. • A specific change thus represents one specific energy, one specific frequency, and therefore one specific wavelength. Bohr’s Equation … • … allows us to find the energy change (Elevel) that accompanies the transition of an electron from one energy level to another. Initial energy level: Final energy level: –B –B Ei = —— ni2 Ef = —— nf2 • To find the energy difference, just subtract: –B –B 1 1 Elevel = —— – —— = B — – — nf2 ni2 ni2 nf2 • Together, all the photons having this energy (Elevel) produce one spectral line. Example 7.6 Calculate the energy change, in joules, that occurs when an electron falls from the ni = 5 to the nf = 3 energy level in a hydrogen atom. Example 7.7 Calculate the frequency of the radiation released by the transition of an electron in a hydrogen atom from the n = 5 level to the n = 3 level, the transition we looked at in Example 7.6. Pop Quiz! • Calculate the wavelength, in nanometers, that corresponds to the radiation released by the electron energy-level change from ni = 6 to nf = 1 in a hydrogen atom. • B = 2.179 x 10-18J • Elevel = Energy Levels and Spectral Lines for Hydrogen What is the (transition that produces the) longest-wavelength line in the Balmer series? In the Lyman series? In the Paschen series? Ground States and Excited States • When an atom has its electrons in their lowest possible energy levels, the atom is in its ground state. • When an electron has been promoted to a higher level, the electron (and the atom) is in an excited state. • Electrons are promoted to higher levels through an electric discharge, heat, or some other source of energy. • An atom in an excited state eventually emits a photon (or several) as the electron drops back down to the ground state. Example 7.8 A Conceptual Example Without doing detailed calculations, determine which of the four electron transitions shown in Figure 7.19 produces the shortest-wavelength line in the hydrogen emission spectrum. De Broglie’s Equation • Louis de Broglie’s hypothesis stated that an object in motion behaves as both particles and waves, just as light does. • A particle with mass m moving at a speed v will have a wave nature consistent with a wavelength given by the equation: l = h/mv • This wave nature is of importance only at the microscopic level (tiny, tiny m). • De Broglie’s prediction of matter waves led to the development of the electron microscope. Example 7.9 Calculate the wavelength, in meters and nanometers, of an electron moving at a speed of 2.74 × 106 m/s. The mass of an electron is 9.11 × 10–31 kg, and 1 J = 1 kg m2 s–2. Uh oh … • de Broglie just messed up the Bohr model of the atom. • Bad: An electron can’t orbit at a “fixed distance” if the electron is a wave. – An ocean wave doesn’t have an exact location—neither can an electron wave. • Worse: We can’t even talk about “where the electron is” if the electron is a wave. • Worst: The wavelength of a moving electron is roughly the size of an atom! How do we describe an electron that’s too big to be “in” the atom?? Electrons – Waves and particles • Remember where we are: • Electrons can behave as BOTH waves and particles. • Louis de Broglie suggested that electrons could gain specific amounts of energy (quanta) based on specific frequencies of light. De broglie • de Broglie also suggested that electrons have other “wave-like” properties. • #1. Electrons can be bent, or diffracted, like light waves. • #2. Electrons bumping into each other can cause interference, when beams of electrons overlap. • Interference can cause either a reduction in energy or an increase in energy. Heisenberg’s Uncertainty principle • In 1927, German physicist Werner Heisenberg helped answer the following question: • If electrons are both particles and waves, then where are they in the atom? • Heisenberg Uncertainty Principle: it is impossible to determine, simultaneously, both the position and the velocity of any particle, including an electron. Schrodinger Wave Functions • Erwin Schrodinger, an Austrian physicist, used this information, along with some “hard-core” calculus, to help describe 4 characteristics of the electron. • He called this – Quantum theory. Electron Characteristics • Before we discuss the 4 characteristics, we need to discuss the probability of finding an electron. • Where would you bet an electron would most definitely be found? Least found? • Orbital – a 3-D region around the nucleus that indicates the probable location of an electron. 4 Characteristics • The 4 characteristics of an electron are known as the 4 quantum numbers. • Characteristic #1: The principle quantum #. • The principle quantum number (n) indicates the main energy level occupied by an electron. • Can only be a positive integer (n = 1, 2, 3, 4, …) 4 Characteristics (cont.) • Characteristic #2: The angular momentum quantum #. • The angular momentum quantum # (l) indicates the shape of the orbital. l = 0, 1, 2, ….,(n-1) • • • • Shape #1: Shape #2: Shape #3: Shape #4: s – spherical p – dumbbell or figure-8 d – cloverleaf f 4 Characteristics (cont.) • Characteristic #3: The magnetic quantum #. • The magnetic quantum # (m) indicates the orientation (+ or -) of an orbital around the nucleus. m=0, +/-1, +/-2,….+/-l • Characteristic #4: The spin quantum #. • The spin quantum # indicates whether an electron in an orbital spins clockwise or counterclockwise. # of ELECTRONS per energy level • We can determine the # of electrons per energy level using the following formula: • 2n2 (where n is the energy level) • When n = 1, there are 2 electrons. • When n = 2, there are 8 electrons.