* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ATOMIC STRUCTURE
Survey
Document related concepts
Transcript
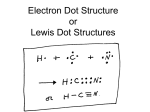
Warm Up….complete on the top third of page 9 in your notebook 1. Calculate the number of each subatomic particle for the following elements: Yttrium (Y element 39) Iridium (Ir element 77) Silver (Ag element 47) 2. Review your venn diagram on page 3. Add additional information based on our lesson last class period. Homework: Complete w/s 35% Date Session # 9/9-10 4 Activity Page # Bohr Model & Lewis Dot Practice 9 Bohr Model & Lewis Dot Diagram Rules 10 Y Ir Ag 8.P.1 Understand the properties of matter and changes that occur when matter interacts in an open and closed container. 8.P.1.1 Classify matter as elements, compounds, or mixtures based on how the atoms are packed together in arrangements. TLW calculate Bohr Model & Lewis Dot model of atomic structure by reading and annotating “rules”, practicing on white boards and completing review w/s. Bringing it all together… For example, OXYGEN is an element on the periodic table. It is made of only one type of atom Actual Oxygen Atom Oxygen on the Periodic Table p8 KING KONG CHEMISTRY Atomic Number = Protons = Electrons Mass Atomic Number = Neutrons Quick Quiz!!! The periodic table has ALL the answers Quiz Powerschool passcode A-Day: FE5QA9F P 10 How are subatomic particles arranged? • Bohr Model of the atom: All of the protons and the neutrons The 3rd ring can hold up to 8 e- 10P 11N The 1st ring can hold up to 2 eThe 2nd ring can hold up to 8 e- ***once a ring is full, it will not give up any electrons in the full ring Valence electrons determine: P 10 •how an atom bonds with other atoms (or if it will bond at all). •atom’s reactivity (how easily it bonds with other elements.) •Atoms with a complete set of valence electrons are stable. They don’t bond with other atoms: don’t gain/lose electrons, don’t share electrons. Word origin/stem late Middle English: from late Latin valentia ‘power, competence,’ from valere ‘be well or strong.’ P9 Draw Bohr models (atomic models) for As a class: • B (Boron, element 5) • Na (Sodium, element 1) On your own: • O (Oxygen, element 8) • Li (Lithium, element 3) Lewis Dot Rules..read and annotate. (mark important text, mark confusing text) Lewis Dot Structures The behavior of an atom is determined by the VALENCE ELECTRONS…so wouldn’t it be easier to just draw those?? (because the others are in full rings and aren’t going anywhere) Write the symbol for the element Calculate the number of valence electrons (Use dots to represent the electrons.) Place electron “dots” around the symbol, starting on the left and moving clockwise. Begin by placing only one electron dot per side of the element symbol. More than 4 valence electrons? Add the second dot to each side (clockwise) Max of two electron dots per side Hydrogen Helium Lithium Berylliu m Boron Carbon Nitrogen Oxygen Fluorine Neon Sodium Magnesi um Aluminum Silicon Phosphor us Sulfur Chlorine Argon