* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Structure of the Atom - Dr. Vernon-
Survey
Document related concepts
Transcript
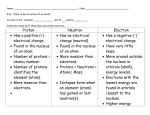
General Chemistry Unit 3 Note Packet – Atomic Structure And The Periodic Table Atom – the smallest part of matter that retains the characteristics of that type of matter considered to be the building blocks of matter atoms consist of a positively charged center, or nucleus, surrounded by negatively charged particles nucleus consists of protons and neutrons negatively charged particles are called electrons existing outside the nucleus in mathematical “regions of probability” particle mass (g) mass (amu) relative charge location in atom proton neutron electron Types of Atoms elements each different type of atom is an element elements (different types of atoms) are distinguished by the # of protons every atom of the same element has the same # of protons (ex: every atom of hydrogen has one proton, every atom of boron has 5 protons, etc.) the # of protons = atomic number elements are organized on the periodic table according to atomic number each element has a chemical symbol the symbol is the first letter of an element’s name capitalized and may have another letter from the name in lower case (examples: C for carbon and Al for aluminum) some symbols are derived from Latin names (example: Argentum is Latin for silver; the symbol for silver is Ag) # protons = # electrons in a neutral atom # protons ≠ # electrons in a charged (positive or negative) atom (called an ion) # neutrons can vary (not the same for every atom of an element) Ions the result of a neutral atom gaining or losing an electron cation positive ion anion negative ion charge of an ion = number of protons – number of electrons Isotopes atoms that have the same number of protons but different numbers of neutrons for example…..hydrogen has three isotopes: hydrogen 1 the atomic mass is “1” which means there is only one proton in the nucleus hydrogen 2 (deuterium) the atomic mass is “2” which means there is one proton and one neutron in the nucleus hydrogen 3 (tritium) the atomic mass is “3” which means there is one proton and two neutrons in the nucleus mass number = # protons plus neutrons atomic mass or atomic weight takes into account all the different isotopes atomic mass/weight is an AVERAGE of all the different isotopes an example calculation for atomic mass is show below: A sample of silver is 51.35% atomic mass/weight? 107 Ag and 48.65% 108 Ag. What is its average Answer: 0.5135 x 107 = 54.9445 0.4865 x 108 = 52.542 Total = 107.4865 amu = average atomic mass/weight (107.49 amu) MODELS OF THE ATOM (1900 to present) Rutherford proposed an atom with a nucleus and negative particles outside of the nucleus. The next major step forward on the model of the atom came after some important breakthroughs in our understanding of electromagnetic energy (light!). light behaves like a wave, but also like a stream of extremely tiny, fast-moving particles (wave-particle duality) light is a form of electromagnetic radiation electromagnetic radiation can be described in terms of: amplitude – height of wave from origin to crest wavelength () – distance between successive crests (meters) frequency () – how fast the wave oscillates; the number of cycles per second (high frequency = high energy) speed of light (c) – 3.00 x 108 meters per second c = Notice: if is large, then must be small since c is constant; the reverse is also true (therefore, long light is low energy while short light is high energy) visible spectrum of light is just part of the electromagnetic spectrum – the part detectable by the human eye the energy absorbed by and given off by objects was thought to be continuous Max Planck, in 1900, proposed that energy is only available in discrete packets, or quanta (singular is quantum); the size of the quantum is related to the frequency of the radiation by a simple equation Bohr Model of the Atom (1913) Bohr applied Planck’s idea of quantized energy to the model of the atom. Electrons move around the nucleus in specific orbits, or energy levels. These energy levels are said to be quantized. An atom has several orbits, each representing a specific energy level. The energy levels are like steps of a ladder. Only specific energy values (steps) can exist – there are none in between. When an atom is not excited (ground state), its electrons are in orbits close to the nucleus. The electrons are at the lowest energy level. If an atom gains energy (excited state), an electron is displaced farther away from the nucleus to one of the higher energy levels. An atom emits energy when an electron falls from a higher energy level to a lower energy level in one sudden drop, or transition. The energy released is electromagnetic energy. The frequency () of the radiation emitted depends on the difference between the higher and lower energy levels involved in the transition. Remember that a specific frequency correlates with a specific wavelength (and color!) through c = . Bohr model of the atom Bohr used this model to explain the discontinuous line spectrum of excited hydrogen atoms. Electron is close to the nucleus of the atom PE is low Electron is further away from the nucleus higher PE Absorption of energy increases the PE of the electron as it moves further away from the nucleus. Thus, an electron of an excited atom absorbs energy in the form of heat or electricity when it moves to a higher energy level. When the electron returns to ground state, it emits the energy it absorbed. The packet of energy emitted corresponds to a specific color on the line spectrum. Problems with Bohr’s Model: 1. it only explained the line spectrum for hydrogen 2. he couldn’t explain why the electrons, which he pictured as circling the nucleus like tiny planets, did not fall into the nucleus every time they emitted light Wave/Particle Paradox: Light sometimes acts as a particle and sometimes acts as a wave. Thomas Young noticed that when electrons are forced through a narrow slit, a pattern of wave interference emerged. He was familiar with Rutherford’s experiment that showed electrons are particles and needed to reconcile the two points of view. This led to the development of the Quantum Mechanical Model of the atom. The Quantum Mechanical Model is based on complex mathematical equations developed by Erwin Schrodinger that describe the wave nature of the atom and the locations of the electrons. Bohr Model vs. Quantum Theory Bohr nuclear model electrons orbit the nucleus like planets around the sun electrons are treated as a particle electrons’ energy is quantized Quantum Theory nuclear model electrons occupy orbitals which are probability spaces described by a mathematical equation electrons are treated as a wave electrons’ energy is quantized QUANTUM THEORY based partly on Heisenberg’s Uncertainty Principle the position and the momentum of a moving object cannot simultaneously be measured and known exactly there is an inherent limitation to knowing both where a particle is at a particular moment and how it is moving in order to predict where it will be in the future an electron is in an orbital (electron cloud) – probability space where an electron can be found a certain percentage of the time as defined by Schrodinger’s equations Schrodinger’s equations Schrodinger’s equations are like your class schedule on file in the office the equations tell us where we are most likely to find an electron your schedule tells us where we are most likely to find you during, for example, 2nd period there is no guarantee you will be in your 2nd period classroom there is no guarantee that the electron will be in particular location at a particular time there is information on your schedule that help us find you there is information derived from Schrodinger’s equations (once all the mathematics has been done) that describes where a particular electron is likely to be found FOUR QUANTUM NUMBERS – information to describe the location of an electron Principal quantum number (n) values = 1,2,3,4,5,6,7 describes the approximate distance of the electron from the nucleus and therefore its energy 2 maximum capacity for an energy level = 2n Sublevel quantum number (l) values = 0,1,2,3…n-1 if n = 1 l = 0 (there is only one sublevel possible) if n = 2 l = 0,1 (there are two sublevels) if n = 3 l = 0,1,2 (there are three sublevels) if n = 4 l = 0,1,2,3 (there are four sublevels) describes the shape of the electron cloud if l = 0 then the orbital shape is spherical (called the s sublevel) if l = 1 then the orbital shape is like a dumb-bell (called the p sublevel) insert diagram if l = 2 then the orbitals are shaped is like… (called the d sublevel) if l = 3 then the orbitals are shaped like… (called the f sublevel) Magnetic quantum number (lm) values depend on l describes the orientation of the electron cloud in space for l = 0, lm = 0 (no way to describe the orientation of a sphere) for l = 1, lm = -1,0,1 (dumb-bell is along the x, y, or z axis) for l = 2, lm = -2,-1,0,1,2 Spin quantum number (ls) values = + ½ or – ½ describes the two possible orientations of the spin axis of an electron electrons act as though they are spinning on an axis like Earth spins on its axis generates an electric field for two electrons to occupy the same orbital, they must have opposite spins DESCRIBING THE LOCATION OF ELECTRONS IN AN ATOM: Aufbau Principle: Electrons are added one at a time to the lowest energy orbitals available until all the electrons of the atom have been accounted for. Pauli Exclusion Principle: No two electrons in an atom can have the same set of four quantum numbers. Taken together, the four quantum numbers describe the state of a particular electron. For example, an electron may have these quantum numbers: 1,0,0,-1/2 (corresponding to n,l,lm, and ls). Think of these as a social security number for an individual, or a street address for a house. No two are identical. Hund’s Rule: Electrons occupy equal-energy orbitals so that a maximum number of unpaired electrons results. In the 2p orbital with three equal energy orbitals, for example, electrons go into 2p orbitals one by one until all three orbitals have one electron. The next three electrons are then paired with the previous three unpaired electrons.