* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download What is Matter - watertown.k12.wi.us
X-ray photoelectron spectroscopy wikipedia , lookup
Bose–Einstein condensate wikipedia , lookup
Heat transfer physics wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Atomic orbital wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Degenerate matter wikipedia , lookup
Electron scattering wikipedia , lookup
Chemical bond wikipedia , lookup
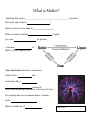
What is Matter? Anything that can be__________________, __________________, touched… Has mass and volume ( ) Matter exists in some state or _________________ Phases of matter include , liquid, gas, non- , & plasma Common of Matter: Solid, Liquid, Gas Non-Newtonian substances sometimes behave like a and sometimes like a consist of freely moving charged particles, (electrons & ions) It is perhaps the most common phase of matter in the Matter is made up of … What is this? What’s an ? The word ATOM (átomos) was first used by the Greek philosopher Atom translates to “ ” It is the smallest particle characterizing an We can’t see the parts of atoms, even with modern technology, so we have an Who is this? When did he live? A theory is a good, logical about something but it hasn’t been proven to be true Atomic Theory It is thought that atoms are made of these common particles: • PROTON- has a positive Charge (P+) • NEUTRON- has a neutral charge; has both positive and negative attributes (N=) • ELECTRON- has a negative charge (e-) Atomic Structure Periodic Table First organized using the known from other chemists like Stanislao Cannizzaro created the table of elements in 1869 of elements Organized horizontally Indicates # of electron Who is this? of elements Organized vertically Indicates # of electrons in Elemental Symbol (Often from Latin or Greek) (1st letter is upper case, 2nd is lower case) Nitrogen (# of P+) 7 N 2 5 (# of P+ & N=) 14.0067 Q: How can I calculate the number of neutrons in an element?? A: Elemental Names & Symbols Iron; Latin Ferrum meaning “firm” Copper; Greek for island of Cypress/ Cuprius Sodium; Natrium (Latin) meaning “soda”/ “salt” Silver; from Latin Argentum meaning “bright” Gold; (Latin) Aurum- Roman Goddess of Dawn Mercury; Greek Hydragyrium meaning ‘liquid silver’ Lead; Latin for Plumbum; origin of plumber … Elements are individual When join together a forms Elements join forming of a There several ways for molecules to … Bonding Atoms join together to form molecules of a compound through Atoms “prefer” to have e- in the outer cloud In order to become more stable, elements will e- (H2O) is an example of covalent bonding Element? Element? Element? Name this compound: Name the type of bonding: Bonding Occurs with One element an electron while another an electron In the case of Sodium Chloride, an electron and This creates gives up gains one : the Na atom has a charge & the Opposite charges and a atom has a negative charge is formed Element? Element? Name this compound: Name the type of bonding: