* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Neuroendocrine tumor wikipedia , lookup
Hormone replacement therapy (menopause) wikipedia , lookup
Bioidentical hormone replacement therapy wikipedia , lookup
Hormone replacement therapy (male-to-female) wikipedia , lookup
Growth hormone therapy wikipedia , lookup
Signs and symptoms of Graves' disease wikipedia , lookup
Hypothalamus wikipedia , lookup
Hypopituitarism wikipedia , lookup
All the information in the slides is included. Slides 17, 21, 23, 24, 30, 31, and 37 have extra pictures.
Biochemical Aspects of Thyroid Hormone
Metabolism
Overview
The thyroid gland is located immediately below the larynx, on each side of and
anterior to the trachea.
Its butterfly shaped, having two lobes connected by an isthmus.
It's important to know the anatomical position and relations of the thyroid gland
when performing surgery. If there is excessive activity of the thyroid gland a
thyroidectomy (surgical removal of all or part of the thyroid gland) may be
performed.
The thyroid gland lies in the vicinity of:
1) The parathyroid gland, which secretes PTH.
2) The recurrent laryngeal nerve.
During a thyroidectomy the surgeon may damage either one. Ifit’s the
parathyroid gland, there will be a decrease in the PTH levels leading to
hypocalcemia. If it’s the recurrent laryngeal nerve the patient’s voice will
become hoarse.
Thyroid gland is one of the largest endocrine glands, normally weighing 15 to 20
grams in adults.
The thyroid secretes two major hormones, thyroxine and triiodothyronine,
commonly called T4 and T3, respectively. About 93% of the metabolically active
hormones secreted by the thyroid gland is thyroxine (T4), 7% is triiodothyronine
(T3). Many endocrinologists consider T4 to be a pre hormone because almost all
of it is eventually converted to T3 in the peripheral tissues by deiodination
reactions.
This reaction is catalyzed by selenium-containing enzyme iodothyronine
deiodinases.
Deiodination can also produce reverse triiodothyronine (rT3)
(physiologically inactive)
NOTE: Of the thyroid hormones, T4 is most secreted, T3 is most active
*Calcitonin is secreted by the para-follicular cells of the thyroid. Like PTH it’s
involved in calcium metabolism, but its function is opposite to that of PTH.
1
Differences between T4 and T3
They differ in rapidity and intensity of action.
Triiodothyronine (T3) is about four times as potent as thyroxine (T4).
Triiodothyronine is present in the blood in much smaller quantities (smaller
concentration)
T3 has a shorter half-life than T4
Congenital Hypothyroidism
• Thyroid hormone is critical for the neurologic development of the fetus.
• Congenital hypothyroidism occurs in 1 of 4,000 live births.
• If the mother has normal thyroid function, the fetus is protected during
development by maternal thyroid hormone crossing the placenta.
• Postpartum, newborns require initiation of appropriate doses of thyroid hormone.
If they do not receive them, neurologic development and growth will be impaired
(stunted). They will suffer from Cretinism
• Screening tests are performed on all newborns to diagnose congenital
hypothyroidism and prevent complications. These are done at the same time as
phenylketonuria (PKU) tests.
Note: In the past, before salt was iodized, some mothers suffered from iodine
deficiency. Because iodine is very important for the functioning of the thyroid
gland (it is the only gland that uses iodine!) its deficiency during pregnancy can
cause maternal and fetal hypothyroidism and impair neurological development of
the fetus. 1
1
During the lecture the doctor said that this was the cause of congenital hypothyroidism, however congenital
hypothyroidism is mostly caused by thyroid dysgenesis (85%) or errors in thyroid hormone biosynthesis (10-15%).
Maternal iodine deficiency will result in transient congenital hypothyroidism -Wiki
2
Cretinism
Children who suffer from Cretinism lack the necessary thyroid hormone in utero or
shortly after birth.They have myxedema(severe hypothyroidism).
* Retarded (stunted) physical and mental development.
They suffer from mental retardation.
* Permanent neurological damage is evident
* Ossification of bone is delayed
* Tooth development is poor, tooth eruption is delayed
* Skin and lips are thick, face is broad and puffy, nose is
flat, and the tongue is enlarged
*Others: clinically the infant is dull and apathetic, body temperature usually below
normal
Chemical Structures
• Deiodinase reactionscan convert T4
into reverse triiodothyronine (rT3)
(physiologically/metabolically inactive)
and T3 in the peripheral tissues.
• T4 and rT3 are inactive. T3 is the
most potent, most active, and has a
short half-life.
• The Doctor wants you to know the
structures.
3
Histology of the thyroid gland
• The thyroid gland is composed of
closed follicles filled with a secreted
material (colloid).
• Follicles are lined with cuboidal
epithelial cells that secrete colloid
•
Colloid is mainly composed of thyroglobulin (a large glycoprotein only found in
the thyroid gland).This glycoprotein is branched, and its branches contain tyrosine
(amino acid). Thyroid hormones are all derivatives of tyrosine; they are
synthesized on the tyrosine residues of the branches of the glycoprotein.
•
Thyroid hormones form within the thyroglobulin molecule. They remain a part of
thyroglobulin during synthesis and later on during storage. (In the colloid)
Major Actions of thyroid hormones
Thyroid hormones are essential for normal growth and development and have many
effects on metabolic processes. Their overall effect on metabolism is to stimulate the
basal metabolic rate, oxygen consumption and heat production. That’s why excessive
secretion of thyroid hormones leads to……Increased appetite, Weight loss, muscle
wasting and heat intolerance
{They induce an increase in net CATABOLISM}
They stimulate the synthesis of a number of hormones and enzymes. One such enzyme
is cytochrome c oxidase, a part of the electron transport chain. The ETC is found in the
inner mitochondrial membrane, and its function is ATP synthesis. An increasein
thyroid hormone will lead to an increase in ATP production, and since ATP production
is associated with heat production patients with hyperthyroidism will suffer from heat
intolerance.
Thyroid hormones increase the sensitivity of the cardiovascular and nervous systems to
catecholamines.This leads to increases in heart rate, cardiac output, and arousal.
NOTE: Hypothyroidism leads to: weight gain (with little increase in eating),
constipation, cold intolerance, depression, and hypercholesteremia.
4
Synthesis of thyroid hormones
1) Iodide trapping: The first stage in the formation of thyroid hormones is transport of
iodides from the blood into the thyroid gland. This is an active process that requires energy.
In the basal membrane there is a sodium/potassium ATPase pump that creates a sodium
concentration outside the cell. A sodium iodine symporter then carries the iodide (along
with sodium) into the follicular cell.The iodide pump concentrates the iodide to about 30
times its concentration in the blood.TSH hormone INCREASES Iodide trapping (more
iodide will increase all the other steps) whereas Thiocyanate Ions DECREASE it
2) Oxidation of Iodide: This is conversion of the iodide ions to an oxidized form of iodine.
Iodide ion is converted to I2 that is capable of combining directly with tyrosine. Oxidation
of iodide is catalyzed by peroxidase/hydrogen peroxide system (a potent system capable of
oxidizing iodides). When the peroxidase system is blocked the rate of synthesis falls down
to zero.
3) Organification: The binding of iodine to the tyrosine residues of the thyroglobulin
molecule is called organification of the thyroglobulin.This process is catalyzed by the
enzyme iodinase.Tyrosine is first iodized to monoiodotyrosine (MIT)(one iodine molecule
on the tyrosine) and then to diiodotyrosine (DIT)(2 iodine molecules on the tyrosine).
5
4) Coupling:Iodotyrosine residues (MIT&DIT) are coupled with one another. The coupling
reactions result in the formation of thyroid hormones T4 and T3
MIT (has 1 iodine molecule) +DIT (has 2 iodine molecules) = T3 (has 3 iodine molecules)
DIT (has 2 iodine molecule) +DIT (has 2 iodine molecules) = T4 (has 4 iodine molecules)
*after coupling the hormone still hasn’t been released! It’s still attached to the
thyroglobulin in the colloid!
NOTE:Each molecule of thyroglobulin contains about 70 tyrosine amino
acid residues.
Under normal condition 70% of tyrosine residues of thyroglobulin are in the
form MIT and DIT and 30% as thyroxine (T4) (only a minor part is in the
form of T3)
*Propylthiouracil (PTU) inhibits peroxidase enzyme as well as coupling of iodinated
tyrosine residues(Steps 2&4), that’s why it can be used in the treatment of patients with
excessive thyroid function. In the figure PTU is the thioamide.
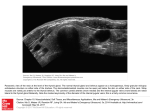
Radioactive iodine uptake (RAIU) can help
(1)diagnose&(2)treatthyroid disease
This is a hot nodule,this area has increased
radioactiveiodine uptake.
(1)Diagnosing an over active thyroid
gland using RAIU.
Graves’ disease: An autoimmune disease that
leads to the production of thyroid stimulating
immunoglobulins. Like thyroid stimulating hormones,
these immunoglobulins bind to the TSH receptor.
Binding results in receptor activation, that results in
increased iodine trapping. When you give a patient
with Graves’ disease a radioactive iodine meal the
gland will appear darker than in the normal scan due to excess iodine uptake.
Multinodular goiter (the thyroid gland works autonomously) & toxic adenoma also
increase the iodine uptake and lead to hot nodules.
6
(2) Treatment using RAIU
Clinicians take advantage of the thyroid glands proclivity to iodine in the treatment of an
overactive thyroid gland. Radioactive iodine can be given to kill the gland tissue.
Surgery and Anti-thyroid drugs like Propylthiouracil (PTU) can also be used.
Storage of thyroglobulin
Thyroid gland can store large amounts of hormone (unlike other endocrine glands).
Each thyroglobulin molecule contains up to 30 thyroxine molecules and a few
triiodothyronine molecules.
The thyroid hormones stored in the follicles are sufficient to supply the body with thyroid
hormones for 2 to 3 months.
When synthesis of thyroid hormone ceases, the physiologic effects of deficiency are not
observed for several months.
Release and secretion of thyroid hormones
1) As a result of TSH stimulation, follicular cells
will extend pseudopods. These pseudopods will
entrap a part of the colloid making an endosome
(pinocytic vesicles). The process by which this
happens is called pinocytosis. The endosome
contains thyroglobulin.
2) A lysosome containing proteases fuses with
the endosome. The proteases cleave MIT, DIT,
T3, and T4 from the tyrosine residues of the
thyroglobulin.Thyroglobulin itself is not released
into the circulating blood.
3) T4 and T3 then diffuse through the base of the
thyroid cell into the surrounding capillaries.
4) Most of the MIT and DIT arerecycled back
into the colloid. Their iodine is cleaved by
deiodinase enzyme that makes iodine available
for making thyroid hormones. Their amino acids
may also be used by the cell. Some are secreted.
7
NOTE: Because TSH stimulates the process of release and secretion of thyroid hormones,the
most important early effect after administration of TSH is to initiate proteolysis of
thyroglobulin. When you give a patient TSH the first thing you will notice is an increase in T3
and T4 levels in the blood. (Within 30 min)
Note: Thyroglobulin is exclusively found in the thyroid gland (It shouldn’t be in the blood serum
or any other tissue). It’s a thyroid biomarker. Its presence in the blood serum may indicate a
thyroid tumor.
Regulation of thyroid hormone secretion:
The Doctor didn’t read the details of each regulator; he said we’ll take them in physiology.
1. TSH
•
TSH (thyrotropin), an anterior pituitary hormone, is chemically a glycoprotein.
•
This hormone increases the secretion of thyroxine and triiodothyronine by the thyroid gland.
•
Specific effects on thyroid gland:
*Increased proteolysis of the stored thyroglobulin.
*Increased activity of the iodide pump.
*Increased iodination of tyrosine to form thethyroid hormones.
*Increased size and increased secretory activity ofthe thyroid cells (hypertrophy).
* Increased number of thyroid cell (hyperplasia).
2. Thyrotropin-Releasing Hormone (TRH)
•
Anterior pituitary secretion of TSH is controlled by a hypothalamic hormone, thyrotropinreleasing hormone (TRH).
•
TRH is secreted by nerve endings in the median eminence of hypothalamus.
•
TRH is transported to the anterior pituitary by hypothalamic hypophysial portal blood.
•
TRH causes an increase in the output of TSH by anterior pituitary.
3. Cold and Anxiety
•
•
Cold is a stimuli, it increases the rate of TRH secretion by the hypothalamus and therefore TSH
secretion by the anterior pituitary gland.
Excitement and anxiety cause an acute decrease in the rate of secretion of TSH.
8
Transport of thyroid hormones
• When released into the circulation, only 0.04% of T4 and 0.4% of T3 are unbound by
proteins (The free T4 and T3 are physiologically active)
• The three major binding proteins, in order of significance, are thyroxine-binding
globulin (TBG), thyroxine-binding prealbumin, and albumin
• The quantity of T4 and T3 in the circulation can be affected by the amount of binding
protein available for carrying these hormones
• High TBG levels (like during pregnancy, due to high estrogen) result in higher levels of
bound thyroid hormones, leading to high levels oftotal T3 and total T4. The total doesn’t
matter though since only the free T3 and T4 are physiologically active!! That’s why you
should measure free not total levels of the thyroid hormones.
*During pregnancy the free hormone levels are normal though the total is elevated
so…… no problem
• Measurement of free T4 and free T3 may be necessary to eliminate any confusion
caused by abnormal binding protein levels.
Mechanism of action of thyroid hormones
Thyroid hormones function through receptors found in
the nucleus. Thyroid receptors, like estrogen and
androgen receptors are part of a superfamily of receptors
called nuclear receptors. This was discovered by
homology of the protein structure at the amino acid level.
Nuclear receptors all have the same domains.
1) DNA binding domain: allows the receptor to bind to
DNA
2) Ligand binding domain: For binding of estrogen,
androgen, or T3
3) Hinge region: allows flexibility of the receptor when
it binds to DNA
9
Though the structure of the nuclear receptors is the same, the mechanism of action of the thyroid
receptor is a little different. There are 2 types of nuclear receptors based on the MECHANISM OF
ACTION.
*Type1 Nuclear receptors: these are usually found in the cytoplasm bound to heat shock proteins.
When the ligand (estrogen or androgen) binds to the receptor: 1) it separates from the heat shock
proteins 2) the nuclear receptor complex goes inside the nucleus 3) it binds to the hormone response
element and4) activates transcription.
This figure represents a Type 2 nuclear receptor
*Type 2 Nuclear receptors: Thyroid receptors on the other hand are ALREADY bound to the DNA.
The free thyroid hormone receptor (TR) without bound hormone is bound to hormone response element
of DNA (HRE). Also bound on the DNA is RXR (Retinoid X receptor) a receptor of the same
superfamily. (TR and RXR are a heterodimer). In the unstimulated state the heterodimer is also bound
to a corepressor (CoR). When the ligand (T3 hormone) enters the nucleus and binds to the heterodimer,
the corepressor is exchanged for a coactivator. The coactivator recruits RNA polymerase which
initiates transcription.
NOTE: Make sure you understand the difference between Type 1 and 2 nuclear receptors, the Doctor
said he’ll ask about them….
10
Thyroid autoantibodies& their relationship to some diseases.
The Doctor read the underlined ones. Focus on the last 2.
Increases in autoimmune hypothyroidism
1
2
3
1) Used as a cancer biomarker.
2) An autoantibody against thyroid peroxide, it binds to thyroid peroxidase and
inhibits its activity. This autoantibody is most commonly seen in Hashimoto's
thyroiditis, the most common cause of hypothyroidism. There is inflammation in
the thyroid gland, and no more thyroid hormone production.
3) An autoantibody against TSH receptor: This autoantibody is most commonly seen
in patients of Graves’ disease, the most common cause of hyperthyroidism. The
thyroid stimulating immunoglobulins bind to TSH receptor and activate it (like
TSH) leading to 1. An increase in the uptake of iodide, 2. Increase production of
T3 and T4, 3.TSH will fall as a result of negative feedback.
Done By: Ranya M. Baddourah
11