* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Igneous Rocks
History of geology wikipedia , lookup
Age of the Earth wikipedia , lookup
Provenance (geology) wikipedia , lookup
Marine geology of the Cape Peninsula and False Bay wikipedia , lookup
Algoman orogeny wikipedia , lookup
Composition of Mars wikipedia , lookup
Clastic rock wikipedia , lookup
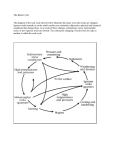
Rock Cycle and Rocks Lab Rocks are aggregates of one or many minerals. Three types of rocks: A. Igneous B. Sedimentary C. Metamorphic Rock cycle is a conceptual model of how all rocks can be formed, transformed, destroyed, and reformed. The rock cycle is driven by earth processes such as atmospheric cycles, oceanic circulation and especially plate tectonics A. Igneous Rocks Igneous rocks form when molten rock (rock liquefied by intense heat and pressure) cools to a solid state. Intrusive igneous rocks crystallize or solidify within the earth’s crust. Magma is molten rock (liquid or liquid/crystal “mush”) that exists below earth’s surface (when cooled it forms rocks such as granite, diorite or gabbro). Extrusive igneous rocks are erupted in a molten state on the earth’s surface and then cool and solidify. Volcanic processes associated with this are natural hazards or disasters. Lava is molten rock flowing out of fissures or vents at volcanic centers (when cooled they form rocks such as basalt, rhyolite, or obsidian). Pyroclastic deposits are accumulations of fragmented material (e.g. ash, bombs, tuffs and volcanic breccias) ejected during volcanic eruptions. Texture is a description of a rock’s constituent parts in terms of their sizes, shapes and arrangement. Rule of Thumb: The size of mineral crystals in an igneous rock may indicate the rate at which the lava or magma cooled to form a rock. Crystal size can also be affected by the amount of gases or the availability of the chemicals in the molten rock that are required to form the crystals. Larger crystals generally indicate intrusive igneous rocks. Smaller crystals generally indicate faster cooling associated with extrusive igneous rocks. Types of igneous rock textures: 1. Aphanitic: fine-grained, less than 1 mm, grains not seen with unaided eye 2. Phaneritic: “coarse grained”; visible crystals; 1 to 10mm 3. Pegmatitic: “very coarse grained”; > 1 cm 4. Porphyritic: composed of both large and fine-grained crystals, and the large crystals are called phenocrysts, and the background is the matrix 5. Vesicular: rocks that have vesicles, resembling a sponge (e.g. scoria and pumice) 6. Pyroclastic: fragmented, angular grains ejected during eruption (e.g. volcanic breccia) 7. Glassy: when lava cools quickly, there is not enough time for large mineral crystals to form (e.g. obsidian) Igneous Rock Mineral Compositions (Assemblages) 1. Felsic: generally the lighter-colored igneous rocks; enriched in silica and aluminum bearing minerals (e.g. quartz and potassium feldspar). 2. Mafic: generally the darker-colored igneous rocks; enriched in magnesium and iron bearing minerals (e.g. olivine and pyroxene). Fig. 1: Bowen’s Reaction Series. The diagram suggests the sequence in which minerals crystallize from an average magma in the asthenosphere when it is slowly cooled. Note the relationship between temperature, crystallization of specific minerals, type of magma, and rocks formed when the magma is cooling. (This is approximate because pressure also affects mineral composition and stability). Viewing the diagram in reverse (bottom to top) also suggests the sequence in which minerals will melt to form magma when rocks are heated. Once these rocks are exposed at the surface, the stability of minerals is greatest for those that formed at lower temperatures, therefore, olivine, calcic plagioclase pyroxene, will weather first whereas quartz is one of the most stable minerals at the surface under weathering conditions. Fig. 2: Mineral composition and color index of igneous rocks. Table 1: List of the rock types supplied for his lab: 1 Granite 5 ignimbrite 6 diorite 8 gabbro 10 peridotite 2 rhyolite 3 obsidian 4 pegmatite 7 andesite 9 basalt Questions and assignments: 1. Take the samples # 1, 2, 3, and 4. Inspect them carefully. What is the mineral content of samples 1,2, and 4? Figures 1 & 2 will be useful for this question! (sample 3 is glassy, thus it is not composed of any minerals!) 1 granite 2 rhyolite 4 pegmatite 2. Now try the same with samples 8, 9, and 10. Enter your results in the table below. 8 gabbro 9 basalt 10 peridotite 3. What about samples 6 and 7? Which minerals would you expect in them? How melts are formed Melts on earth are being generated at very specific sites in the crust and there they are of a very distinctive composition. Until the advent of the concept of global tectonics, this was a problematic topic. Global tectonics offers an excellent concept how melts are being generated. In a nutshell, at mid-ocean ridges, mafic melt wells up and emerges on the sea floor as the ocean plates are spreading. This melt forms basaltic or gabbroic rock, depending on the depth below the sea floor. This also means, that the sea floor is expanding and if this is happening, someplace else, crustal material has to make space for this! This is happening at subduction zones where oceanic crust is thrust deep into the mantle where it melts again. Wet sea floor sediments will release the water content and this water under pressure aids melting. It also ends up in the melt and if this melt gets to the surface, it is released as steam during an eruption. Notice that the continents don’t appear to get subducted! Continents are mainly rocks that tend to be composed of more felsic rocks (such as granites, diorites etc.). Among the places where this melt will appear again is at active continental margins or at island arcs. But this melt has undergone some evolution and is now less mafic. The original basaltic melt is now andesitic. This is named after the Andes Mountains in South America where such an active margin exists. Continent-continent collisions do not lead to subduction but the crust will thicken at those sites, mountains will build and metamorphism of the sediment packages that are now deeper and under higher pressure and temperature will occur. Melting will also occur and produce more felsic melts. 1. Mid-ocean Ridges 5. Back-arc Basins 2. Intracontinental Rifts 6. Ocean Island Basalts 3. Island Arcs 7. Miscellaneous IntraContinental Activity u kimberlites, carbonatites, anorthosites... 4. Active Continental Margins Fig. 3: The locations on the earth’ crust where melt is generated. Fig. 4: melt being generated at a subduction zone. Here aslab of ocean floor subducts underneath a conitinent. Fig, 5: Continent-content collision. No subduction occurs but the crust is thickening and metamorphism occurs as well as melting of continental rocks leading to a more gfelsic melt. 4. Why do continents appear to float on top and don’t subduct? To find out, let us try an experiment: Last week, we determined the density of minerals by weighing them in air and in water and thus determining the density. This week, we will use a sample of granite and one of basalt and measure the density. If you are not certain how we did this, the last page gives you the steps once more. What explanation can you come up with to explain the behavior of the continental plates with respect to the oceanic plates? Fill in the densities you determined into the table . Basalt Granite Density measured 5. Does your answer of question # 4 fit well with the results of questions 1 and 2 as well as what you learned last week about mineral properties? Why? The table of the mineral properties is included once more below. 6. A final experiment: use sample 11 and a container with water. This is a sample of pumice, a volcanic rock. Does it float or sink? Why? (Hint: What do you notice when you inspect it more closely?) Density, g/cm3 Cleavage/ fracture Hardness (Mohs) Color Streak Luster Quartz 2.65 No cleavage, irregular fracture 7 white nonmetallic Potassium feldspar 2.54-2.62 2 perfect cleavage planes at near 90° 6 colorless, white, brown, pink. purple white, pink, gray, green white nonmetallic Plagioclase feldspar 2.62-2.76 2 perfect cleavage planes at near 90° 6 white, gray, colorless white nonmetallic Biotite 2.8-3.2 1 perfect cleavage 2.5-3 2.76-2.88 2.5-3 Amphibole (hornblende) 3.0-3.4 nonmetallic 3.2-3.4 white to off-white nonmetallic Olivine 3.27-4.37 white nonmetallic Garnet 3.5-4.3 white Calcite 2.7 3 rhombohedral cleavages 3 dark green, black, dark brown black, dark green, brown yellowgreen to olive-green red, redbrown, yellow, black, green Colorless, white, gray, pink, yellowish white to off-white Pyroxene (augite) 1 perfect cleavage 2 perfect cleavages at 60 and 120° 2 perfect cleavages at near 90° no cleavage, irregular fracture no cleavage, irregular fracture white to off-white to tan white nonmetallic Muscovite brown, black, dark green light color Pyrite 5.0 No cleavage 6-6.5 1 perfect cleavage 1 cleavage in coarse metallic hematite 2 No cleavage 6 Gypsum Hematite up to 5.25 Magnetite 5.1-5.2 5-6 5-6 6.5-7 7-7.7 1.5-6 Other Sample # diagnostic properties 1 common with roughly parallel streaks very thin, perfectly parallel lines on some surfaces. very easy to cleave 2 very easy to cleave cleavage angles and dark color cleavage angles and dark color color, lack of cleavage 5 nonmetallic lack of cleavage, hardness 9 white nonmetallic 10 brassy yellow dark gray metallic colorless, white, gray red-brown to gray, depending on coarseness Silvery gray to black white nonmetallic red-brown metallic or nonmetallic Hardness, cleavage, acid response: fizzes Luster, color, hardness Hardness, cleavage Streak color Dark gray metallic or nonmetallic magnetic! 14 nonmetallic 3 4 6 7 8 11 12 13 Density determination: To accomplish this, fill the beaker about ¾ full with water and set it aside. Now turn on the balance and make certain it reads ‘0.00’ and is in the ‘gram mode’, it should display ‘g’ on the upper right of the readout panel. If it does not display ‘g’, hit the mode button until it does. Place the sample on it and obtain its mass. This value is in grams. Next, place the beaker filled with ¾ of its volume in water. Hit the ‘tare’ button, the readout should display ‘0.00’. Use the supplied string, make a slipknot and attach the sample to it. Now suspend the sample in the water, making certain it is completely submerged. Do not let it touch the bottom or the sides of the beaker. Record the number on the readout. This is the mass of the water displaced by the sample! Since the density of water is 1.00 g/cm3, the volume displaced is the same numerical value but it is expressed in cm3. Divide the mass of the sample by the volume to obtain the density of the sample in g/cm3. 1: turn on balance and tare to show ‘0’. 2. Weigh sample 3. Place beaker with water on balance and tare to indicate ‘0’. 4. Suspend sample in water to weigh displaced water