* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download - Lorentz Center
Wave function wikipedia , lookup
Dirac equation wikipedia , lookup
Electron configuration wikipedia , lookup
Coherent states wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Quantum field theory wikipedia , lookup
Tight binding wikipedia , lookup
Bell's theorem wikipedia , lookup
Many-worlds interpretation wikipedia , lookup
Path integral formulation wikipedia , lookup
Orchestrated objective reduction wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Quantum state wikipedia , lookup
Renormalization group wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Scalar field theory wikipedia , lookup
Renormalization wikipedia , lookup
Topological quantum field theory wikipedia , lookup
Matter wave wikipedia , lookup
Hydrogen atom wikipedia , lookup
EPR paradox wikipedia , lookup
Wave–particle duality wikipedia , lookup
Canonical quantization wikipedia , lookup
Atomic theory wikipedia , lookup
Copenhagen interpretation wikipedia , lookup
Interpretations of quantum mechanics wikipedia , lookup
History of quantum field theory wikipedia , lookup
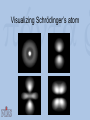
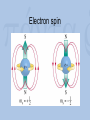
Visualization as a Tool for Scientific Understanding Henk W. de Regt Lorentz Fellow, NIAS 2009-2010 Faculty of Philosophy, VU University Amsterdam Lorentz Workshop Understanding and the Aims of Science Leiden, 4 June 2010 Schrödinger on Heisenberg “I naturally knew about his theory, but was discouraged, if not repelled, by what appeared to me as very difficult methods of transcendental algebra, and by the lack of Anschaulichkeit.” Heisenberg on Schrödinger “The more I think of the physical part of Schrödinger’s theory, the more abominable I find it. What Schrödinger writes about Anschaulichkeit makes scarcely any sense, in other words I think it is crap.” Outline 1. Introduction 2. The loss of visualizability in atomic physics 3. The debate about Anschaulichkeit 4. A theory of scientific understanding Classical physics and Anschaulichkeit C.F. von Weiszäcker (1979): “Physicists identify anschaulich with ‘classical’ because classical physics describes all physical phenomena as states of quantities in threedimensional, Euclidean space and as changes of these states in one-dimensional, objective time.” Bohr’s atomic model (1913) A partially visualizable, semi-classical model: – discrete electron orbits visualizable – ‘quantum jumps’ non-visualizable The waning of visualizability • Wave-particle duality – of light (Einstein 1905) and matter (De Broglie 1923) – no unambiguous visualization • Reality of electron orbits disputed – Pauli’s fourth quantum number (1924) – atoms completely non-visualizable Two new quantum theories • Heisenberg’s matrix mechanics (1925) – only relations between observable quantities – no (visualizable) model of atomic structure • Schrödinger’s wave mechanics (1926) – atomic structure is complex wave phenomenon – no quantum jumps; no wave-particle duality – a promise of visualization Visualizing Schrödinger’s atom Schrödinger on Anschaulichkeit “The aim of atomic research is to fit our empirical knowledge concerning it into our other thinking. All of this other thinking, so far as it concerns the outer world, is active in space and time.” “We cannot really alter our manner of thinking in space and time, and what we cannot comprehend within it we cannot understand at all.” The epistemic power of visualization Examples: • Wave mechanics yielded many more applications than matrix mechanics. • Electron spin (Goudsmit and Uhlenbeck, 1925): visualizing Pauli’s fourth quantum number. • After WW II: Feynman diagrams Electron spin Wolfgang Pauli (1900-1958) Pauli on Anschaulichkeit “We should not want to clap the atoms into the chains of our preconceptions […] but we must on the contrary adjust our ideas to experience.” “Even though the demand […] for Anschaulichkeit is partly legitimate and healthy, still this demand should never count in physics as an argument for the retention of fixed conceptual systems. Once the new conceptual systems are settled, then also these will be anschaulich.” Heisenberg’s 1927 paper: Über den anschaulichen Inhalt der quantentheoretischen Kinematik und Mechanik • Reinterpretation of Anschaulichkeit “We believe we understand [anschaulich zu Verstehen] a physical theory when we can see its qualitative experimental consequences in all simple cases and when at the same time we have checked that the application of the theory never contains inner contradictions” • Uncertainty relations: Δp.Δq ≥ ½(h/2π) Outcome of the debate • Schrödinger’s attempted visualization of atomic structure failed • Heisenberg admitted visualizable concepts • Result: new ‘quantum mechanics’ that combined both theories Conclusions of case study • Visualizability problematic in quantum domain can’t be a necessary condition for intelligibility. • But visualization remained a useful tool for understanding, even in the quantum era. The epistemic significance of intelligibility • Thesis: intelligibility is epistemically significant. • Intelligibility = pragmatic understanding of theory, ability to work with the theory. • Explanations (understanding of phenomena) require intelligible theories. Explanation and pragmatic understanding • Deductive-nomological (Hempel) – Example: flying jets – Deductive reasoning requires skill • Model-based (Cartwright) – No deduction, but tinkering (approximation, idealization) – Skills and judgment needed Intelligibility Positive value that scientists attribute to the cluster of theoretical qualities that facilitate use of the theory – No intrinsic property, but related to skills context-dependent – Examples of valued qualities: visualizability, causality, unifying power, simplicity, etc. A possible test for intelligibility (esp. physical science) A scientific theory is intelligible for scientists if they can recognize qualitatively its characteristic consequences without performing exact calculations. Conclusion: understanding as a means and an end • Epistemic aim of science: explanations that provide understanding of phenomena. • Understanding phenomena requires understanding theories – intelligibility! • Intelligibility: not a matter of ‘feeling good’ about a theory, but of being able to use it. If you want more: H.W. de Regt, S. Leonelli & K. Eigner (eds), Scientific Understanding: Philosophical Perspectives. University of Pittsburgh Press, 2009. H.W. de Regt & D. Dieks, ‘A contextual approach to scientific understanding’, Synthese 144 (2005) 137-170. H.W. de Regt, ‘Making sense of understanding’, Philosophy of Science 71 (2004) 98-109.