* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Hypoplastic left heart syndrome
Cardiac contractility modulation wikipedia , lookup
Cardiovascular disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Electrocardiography wikipedia , lookup
Heart failure wikipedia , lookup
Echocardiography wikipedia , lookup
Marfan syndrome wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Turner syndrome wikipedia , lookup
Coronary artery disease wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Aortic stenosis wikipedia , lookup
Myocardial infarction wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Atrial septal defect wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Congenital heart defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
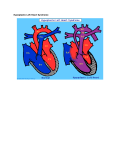
Ultrasound Obstet Gynecol 2000; 15: 271±278. Editorial Hypoplastic left heart syndrome J.M. SIMPSON INTRODUCTION Hypoplastic left heart syndrome (HLHS) is the term used to describe a group of congenital heart lesions characterized by severe underdevelopment of left heart structures. Up to the early 1980s this condition was untreatable and was uniformly fatal in early infancy. The initial reports of Norwood and colleagues provided the impetus for active intervention for this condition1. Advances in surgical techniques mean that intervention is now possible for this condition, with better results in recent years2. Hypoplastic left heart syndrome may be diagnosed during fetal life, thus a knowledge of this condition is highly relevant to those involved in prenatal diagnosis. This Editorial aims to describe the antenatal sonographic features of HLHS and to address issues regarding prenatal and postnatal management. DEFINITION Hypoplastic left heart syndrome is not a single entity but is rather a spectrum of congenital heart malformations characterized by severe hypoplasia of the left ventricle and left ventricular outflow tract. In `classical' HLHS the aortic valve is atretic, with either atresia or severe hypoplasia of the mitral valve. In some instances, the term has been applied to other lesions including critical aortic stenosis with severe hypoplasia of the left ventricle, unbalanced atrioventricular septal defects with hypoplasia of the left ventricle and aorta, and severe coarctation of the aorta. In this Editorial, unless stated otherwise, the term HLHS will be applied to cases of `classical' HLHS. Where relevant, reference will be made to the other variants of HLHS. SO NOGR A PH IC FEAT URES The sonographic features of some of the more common forms of HLHS are described below. `Classical' hypoplastic left heart syndrome Mitral and aortic atresia There is no communication between the left atrium and left ventricle. This may be due to a complete absence of the left atrioventricular connection or an imperforate mitral valve. Typically, the left ventricle is slit-like and there may be no demonstrable left ventricular cavity at all (Figure 1). Aortic atresia (mitral valve patent) INCIDENCE The birth incidence of HLHS has been estimated to be 0.1± 0.25/1000 livebirths3,4. These birth estimates will certainly underestimate the incidence during fetal life, because they do not include cases of spontaneous intrauterine death which occur with increased frequency in fetuses with congenital heart disease. One large study described a 5% rate of spontaneous intrauterine death in a series of 161 cases of HLHS diagnosed prenatally5. EDIT ORIAL The left ventricle is echogenic, hypoplastic and globular, contrasting with the slit-like ventricle observed in mitral atresia (Figure 2). Other variants of HLHS Critical aortic stenosis with hypoplastic left ventricle In this condition, the left ventricle typically appears 271 Editorial Figure 1 Mitral and aortic atresia. The right atrium and right ventricle are easily seen but the left ventricle is exceedingly hypoplastic with no demonstrable cavity. RA right atrium; RV right ventricle; LA left atrium. echogenic and poorly contracting. The mitral valve is patent and there is some forward flow of blood through the stenotic aortic valve. Sequential prenatal echocardiographic studies usually demonstrate subnormal growth of the left heart with advancing gestation so that, by term, the left ventricle and aorta may be severely hypoplastic. In such cases, the left heart structures may be judged incapable of supporting the systemic arterial circulation and management will be akin to other forms of HLHS6,7. It is in this group of fetuses, with severe aortic stenosis, that balloon dilation of the aortic valve has been advocated, with the aim of promoting growth and function of the left ventricle, thereby maximizing the possibility of a biventricular repair8,9. In one case in which prolonged survival Figure 2 Aortic atresia (mitral valve patent). The right ventricle forms the apex of the heart. The left ventricle is globular, echogenic and hypoplastic. RV right ventricle; LV left ventricle. 272 Simpson Figure 3 Critical aortic stenosis with hypoplastic left ventricle. The right ventricle forms the apex of the heart. The left ventricle is hypertrophied, globular and mildly echogenic. RV right ventricle; LV left ventricle; RA right atrium. was documented, prenatal echocardiographic measurements did not demonstrate any short-term improvement in cardiac function in that fetus7. Thus the role of prenatal intervention in such cases remains controversial (Figure 3). Severe coarctation of the aorta Features of coarctation of the aorta in the fetus include a marked dominance of right heart structures compared to those of the left, affecting the great arteries and/or ventricles coupled with relative hypoplasia of the distal aortic arch. In these fetuses the mitral and aortic valves are patent. The diagnosis of coarctation of the aorta is Figure 4 Severe coarctation of the aorta. This echocardiogram was taken at 27 weeks gestation. There is marked asymmetry of ventricular and atrial size. The left ventricle does, however, extend to the apex of the heart and the mitral and aortic valves were patent. By term, the left ventricle and aorta were so hypoplastic that a Norwood strategy was adopted postnatally. LV left ventricle; LA left atrium; RV right ventricle; RA right atrium. Ultrasound in Obstetrics and Gynecology Editorial Figure 5 Severely unbalanced atrioventricular septal defect. The right ventricle is dominant and the left ventricle is tiny and slit like. This fetus also had interruption of the inferior vena cava (left atrial isomerism/visceral heterotaxy). RV right ventricle; RA right atrium; LV left ventricle. particularly difficult during fetal life and sequential studies may be required to be confident of the diagnosis10±12. In most of these cases, surgery on the aortic arch is sufficient for a biventricular repair of the heart. In a subgroup, however, the left ventricle, mitral valve and aorta are so hypoplastic that the management of more classical forms of HLHS may be adopted postnatally. This poses major problems for counselling of parents with affected fetuses early in pregnancy. Parents of fetuses with severe coarctation of the aorta need to be advised that if growth of left heart structures is poor as pregnancy advances, then a biventricular repair may be impossible and the postnatal management will be that of HLHS (Figure 4). Severely unbalanced atrioventricular septal defect Some fetuses with atrioventricular septal defects demonstrate marked asymmetry in the size of the ventricles and great arteries. If the left ventricle and aorta are severely hypoplastic, then it may not prove possible to septate the heart surgically into left and right ventricles, because the left ventricle and aorta are too small for this to be a viable option. In such cases the postnatal management will be that of fetuses with classical HLHS. The discrepancy between right and left heart structures has a variable progression, making prognostication early in pregnancy extremely difficult (Figure 5). A S S O C I AT E D F E AT U R E S The incidence of extracardiac and chromosomal abnormalities in fetuses with HLHS varies widely in reported series. Unfortunately, this often reflects differences in definitions of what constitutes HLHS. Differences in referral population will also influence the incidence of Ultrasound in Obstetrics and Gynecology Simpson extracardiac and chromosomal abnormalities. Previous data from this center has demonstrated a 4±5% incidence of karyotypic abnormalities in classical HLHS, defined as aortic atresia with an atretic or stenotic mitral valve5,13. Others have reported a higher incidence of karyotypic abnormalities in HLHS14±16. In critical aortic stenosis, the incidence of karyotypic abnormalities is exceedingly low with no such abnormalities demonstrated in two large series from this center5,13. Fetuses with atrioventricular septal defects or coarctation of the aorta have a much higher incidence of karyotypic abnormalities5,13. In the study of Natowicz et al. nine of 83 patients with HLHS had chromosomal abnormalities including trisomy 13, trisomy 18 and trisomy 2116. Close inspection of their data, however, reveals that many affected fetuses had unbalanced atrioventricular septal defects or coarctation of the aorta, rather than classical HLHS. Extracardiac structural malformations may coexist with HLHS, which can adversely affect the prognosis17. The incidence of extracardiac abnormalities described in a postmortem series of 128 cases of classical HLHS from our center was 3%18 which is much lower than other series15,16. In terms of management, a detailed search for extracardiac malformations is required in all cases of HLHS, and fetal karyotyping should be discussed. The estimation of risk of karyotypic abnormalities will depend on the exact form of HLHS that is identified. Hence, an accurate cardiological diagnosis is essential. In practice, some parents may elect to terminate the pregnancy on the basis of the cardiac findings, regardless of associated anomalies. In such circumstances, blood or tissue should be sent for fetal karyotyping and a postmortem examination of the fetus may provide additional information. A N T E N ATA L M A N A G E M E N T Most cases of HLHS are detected during midtrimester ultrasound scanning of the low-risk obstetric population. From the parents' perspective, the pregnancy will have been thought to be proceeding normally up to this point and the suspicion of a major cardiac abnormality at this stage of pregnancy is immensely stressful. Urgent referral for confirmation of the diagnosis and an explanation of the prognosis is indicated which will usually involve a fetal cardiologist or a pediatric cardiologist with specific training in fetal echocardiography. At this author's center, all parents are initially seen by one of two consultants accompanied by a nurse counsellor with extensive experience in pediatric cardiology. Information given during the initial consultation is accompanied by written information about the condition. The postnatal treatment options are explained, including surgical and nonsurgical risks, and the uncertain long-term prognosis. Termination of pregnancy is also discussed. In the United Kingdom, the majority of patients opt to terminate the pregnancy following the diagnosis of HLHS19 which is in accord with our own departmental experience. There is also the option of nonintervention following the delivery of affected babies. This final option has become more 273 Editorial controversial, particularly in view of the improving results of postnatal interventions. My own view is that the wishes of the parents should be respected in this regard. We have found that the provision of relevant written information to parents at the initial consultation has been of great benefit. The retention of verbal information given to parents, devastated by the finding of severe congenital heart disease, is likely to be limited. Such information should be coupled with the offer of a repeat consultation if desired20. Some parents will also wish to discuss postnatal management directly with the pediatric cardiac surgeon who will be involved in their child's care. It is vital that there is a co-ordinated team approach to management, which will include the referring obstetrician, fetal medicine specialist, cardiologist, cardiac surgeon and intensive care staff. Some parents will also consult outside sources of information on hypoplastic left heart syndrome, including the internet. This has occurred with sufficient frequency that we now provide the internet addresses of relevant sites, including a national parent support group for HLHS, `Left Heart Matters' (www.lhm.org.uk). P O S T N ATA L T R E AT M E N T S T R AT E G I E S Prenatal recognition of HLHS provides an opportunity to plan postnatal management. This means optimizing the condition of the affected baby to undergo surgical intervention. In the vast majority of cases of HLHS diagnosed prenatally at our center, the parents opt for delivery at the cardiac center to avoid the need for postnatal transfer of the baby and, importantly, to avoid potential separation of the parents and baby. The advantages of this approach have been described previously21. Following delivery, a prostaglandin E infusion is commenced to maintain ductal patency and the prenatal diagnosis of HLHS is confirmed echocardiographically. The circulatory physiology of the newborn infant with HLHS is abnormal. Pulmonary venous blood returns to the left atrium and crosses the atrial septum to the right atrium where it mixes with systemic venous blood. All blood leaves the heart through the right ventricle and main pulmonary artery. By maintaining ductal patency, blood can flow from the main pulmonary artery into the aorta, where a proportion will pass in a retrograde manner into the head and neck vessels, and the remainder will pass to the descending aorta. This circulation presents a challenge in achieving the appropriate balance between pulmonary and systemic blood flow. There are two surgical options. The first option is staged palliative surgery of the type first described by Norwood and colleagues. The second is that the infant undergoes heart transplantation. The details of both management strategies are described below. Norwood Protocol The first Norwood operation was performed in the United States in the early 1980s1. The Norwood approach to 274 Simpson Figure 6 Diagramatic representation of the first stage Norwood operation, Surgery involves (a) reconstruction of the aortic arch, (b) insertion of a systemic to pulmonary artery shunt and (c) atrial septectomy to prevent obstruction of pulmonary venous return and allow mixing of oxygenated and deoxygenated blood. RA right atrium; RV right ventricle; LA left atrium. Diagram reproduced from Left Heart Matters website (www.lhm.org.uk), with permission management of the hypoplastic left heart syndrome is a staged surgical palliation. The first stage, which is usually performed in the first week of life, consists of reconstruction of the aortic arch, using the main pulmonary artery as a single outlet from the right ventricle of the heart (Figure 6). As part of this surgery the branch pulmonary arteries are disconnected from the main pulmonary artery. Therefore, to maintain adequate pulmonary blood flow, a systemic to pulmonary artery shunt is inserted. Pulmonary venous flow returns to the right atrium by crossing the atrial septum from left to right. There is the potential for restriction of blood flow at the level of the atrial septum so an atrial septectomy is routinely performed. This permits mixing of pulmonary venous and systemic venous blood. The second stage of the Norwood protocol involves surgical anastamosis of the superior vena cava to the Figure 7 Second stage Norwood operation. Venous blood flowing through the superior vena cava is redirected to the pulmonary arteries and the systemic to pulmonary artery shunt is taken down. Diagram reproduced from Left Heart Matters website (www.lhm.org.uk), with permission Ultrasound in Obstetrics and Gynecology Editorial Figure 8 Completion of Fontan circulation. Blood flow through the superior and inferior vena cava is directed to the pulmonary circulation. The right ventricle pumps oxygenated blood though the reconstructed aorta. Diagram reproduced from Left Heart Matters website (www.lhm.org.uk), with permission pulmonary arteries (Figure 7). This is typically performed at 6±12 months of age by a hemi-Fontan or bidirectional Glenn operation. The third stage of palliation consists of redirecting flow from the inferior vena cava to the pulmonary arteries and is usually performed beyond the age of two years (Figure 8). This final `Fontan' circulation leaves systemic venous blood passing directly to the pulmonary arteries under passive venous pressure. Oxygenated systemic arterial blood is pumped around the body through a reconstructed aortic arch by the right ventricle. One of the main concerns of parents whose fetus is found to have HLHS is the risk of interventions and the long-term prognosis. The procedural risk with the first stage Norwood operation continues to be much higher than the later stages. Operative survival has ranged from 46 to 76% in a number of reports from centers in the United Kingdom and the United States2,22±25. The review of 212 cases of patients undergoing stage 1 Norwood surgery at the Children's Hospital in Boston demonstrated that survival improved with increased experience of intervention for HLHS2. This is likely to reflect improved surgical technique and perioperative care. The second and third stages of the Norwood protocol have a much lower mortality than the first stage, typically around 5% or less2,22,24,25. The long-term prognosis of the final Fontan circulation is essentially unknown. Data from children with a Fontan circulation following management of a variety of different forms of congenital heart disease has demonstrated decreasing functional status with time which seems to reflect the Fontan circulation per se rather than any specific operative complication26. Given that the Norwood strategy was only devised in the 1980s, many aspects of the long-term prognosis remain unknown. As well as concerns about the mortality of HLHS, there have been legitimate concerns about the morbidity of treatment. In 1995, Rogers et al. reported major developmental abnormalities in seven of 11 survivors of surgery for HLHS27. A later report by a different group Ultrasound in Obstetrics and Gynecology Simpson demonstrated more encouraging results with one of 12 children meeting criteria for significant mental retardation28. In the cohort of 26 survivors of HLHS surgery at the author's center, three have significant neurological impairment24. There are a number of possible causes of neurological impairment in HLHS. Post-mortem data has indicated that congenital brain anomalies may coexist with HLHS29. Some patients have been found to have cerebral ischemic changes or infarcts preoperatively28,30. Improved preoperative condition is one major benefit of prenatal diagnosis of HLHS, which may help to reduce neurological morbidity31. In cases where a diagnosis is not made prenatally, many of these babies present with circulatory collapse which may adversely affect the neurological outcome. Operative factors may also impact on neurological outcome. The study of Kern et al. suggested a relationship between a prolonged circulatory arrest time during surgery and poor intellectual function28. Thus, improvements in surgical technique have the potential to reduce neurological morbidity. Heart transplantation Neonatal heart transplantation is an alternative to staged surgical palliation for the treatment of HLHS. This has the attraction that the circulatory physiology is more normal than the Norwood approach. Various centers have produced encouraging results for this approach with 1year survival of 70±84% in infants who undergo surgery32±34. A better functional status has also been reported in survivors of heart transplantation vs. the Norwood approach35. Although this approach appears attractive, it is severely limited by the shortage of suitable donors. There are also longer term problems including rejection of the transplanted heart, accelerated atherosclerosis and side-effects of immunosuppressive drugs such as infection and lymphoproliferative disease. This technique does, however, present an alternative to the Norwood strategy where there is donor availability. Long-term issues concerning postnatal interventions In a thought-provoking Editorial in this journal 5 years ago, Charles Kleinman described a number of cardiological procedures including the Mustard procedure for transposition of the great arteries, and the Fontan operation, where initial enthusiasm was subsequently tempered by the identification of long-term complications36. That Editorial also summarized the ongoing debate about the `optimal' surgical approach for HLHS. Whatever surgical approach is taken to the management of HLHS postnatally, the interventions are palliative rather than curative. This is a crucial point that must be adequately addressed during discussions with parents both prenatally and postnatally. For fetal and pediatric cardiologists, one of the major challenges is to impart to parents some understanding of the long-term impact of complex congenital heart disease on the child and family. This is often done in the context of 275 Editorial relatively little medium to long-term morbidity data compared to the amount of data on short-term mortality and surgical technique. It is to be hoped that the large surgical series will be followed by an equally rigorous assessment of the nonsurgical mortality and morbidity of children with HLHS. R E S U LT S O F S C R E E N I N G F O R H L H S A recent United Kingdom national study recorded the antenatal detection rates of congenital heart lesions requiring intervention in infancy19. Two-thirds of cases of HLHS were actually detected prenatally during the study period (1993±1995). This was the highest detection rate of any cardiac lesion. Cardiac defects which primarily involved the outflow tracts and were compatible with a normal four chamber view were detected much less frequently, for example only 3% of cases of transposition of the great arteries. Despite the high overall national detection rate of HLHS, there was a large regional variation. The detection rates of HLHS elsewhere do not appear as high as those recorded in the United Kingdom37,38. The differences in detection rates between different countries and regions may be explained, in part, by variations in the uptake of midtrimester anomaly scanning. The training, experience and supervision of those who are performing such scans will also influence detection rates. The quality of ultrasound equipment, the time permitted for sonographic evaluation and the ease with which an expert opinion can be arranged will also be important. When HLHS presents postnatally for the first time, parents increasingly ask whether the lesion `should' have been detected prenatally on routine obstetric ultrasound scans. This is a thorny issue with potentially major medicolegal implications. It is important that parents understand that midtrimester anomaly scans are a form of screening that has a number of purposes including assessment of fetal size, identification of major structural anomalies and a variety of `markers' of chromosomal abnormalities. Thus, examination of the heart is only one component of the assessment of the fetus and an expectation of detection of all major congenital cardiac abnormalities at primary centers is unrealistic. Even at a tertiary fetal cardiology center, we do not offer a 100% guarantee that all forms of congenital heart disease have been excluded. Our understanding of the progression of congenital heart disease has increased in recent years. In aortic stenosis, for example, it is well documented that the rate of growth of the left ventricle and aorta is frequently subnormal7. Thus, an affected fetus with a normal sized left ventricle at 20 weeks gestational age may have a severely hypoplastic left ventricle by term and thus will ultimately demonstrate appearances which fall within the spectrum of HLHS. Also, for fetuses with severe coarctation of the aorta and unbalanced atrioventricular septal defects the asymmetry between left and right heart structures may become more marked with advancing 276 Simpson gestational age10±12. This means that the appearances of the heart when studied at 20 weeks gestation may be considerably different from the appearance at term. D OE S PR E N ATA L D I A G N OSI S OF HL HS AFFECT OUTCOME? The question of whether prenatal diagnosis of HLHS affects the postnatal outcome is controversial. Some studies have compared the early surgical mortality of fetuses who presented prenatally with those who presented postnatally39. The study of Kumar et al.39 compared the early surgical outcome of 27 babies with HLHS diagnosed prenatally with 47 babies in whom the diagnosis was made postnatally. There was no significant difference in operative mortality, but the preoperative condition of the prenatally diagnosed group was better than those diagnosed postnatally. Others have demonstrated a lower surgical mortality in babies diagnosed prenatally31. In the study of Tworetzky et al.31 nine of 10 infants diagnosed prenatally survived first stage Norwood surgery compared with 12 out of 23 infants diagnosed postnatally. One of the difficulties in interpretation of such data is that often no account is taken of babies in whom no diagnosis is made prenatally and who do not survive to reach a tertiary center, or who are in such poor condition that they do not undergo surgery. In order to obtain such data, a detailed registry of deaths within a defined geographical region would have to be available. Such data was produced from the north-east region of England between 1985 and 1990. Twenty-six cases of HLHS were documented during the study period, of which four (15%) were first diagnosed at autopsy4. Thus, surgical series of cases of HLHS diagnosed postnatally represent a selected group of patients which hinders relevant comparison with cases of HLHS diagnosed prenatally. With the trend towards early postnatal discharge of babies from hospital, it is possible that an increasing number of babies with severe congenital heart disease will present at home rather than in a hospital environment, with the potential for a higher rate of `out of hospital deaths'. In the study of Kumar et al. almost a quarter of the postnatally diagnosed cases of HLHS had been discharged from hospital before presentation39. These infants appeared to have the worst metabolic status on admission to hospital. Whilst this did not appear to reduce short-term survival, the effect on long-term neurological development is unknown. In a regional, population-based study in the United Kingdom 94 of 120 babies with left heart obstructive lesions were discharged from hospital prior to presentation40. Thus, prenatal diagnosis of such conditions may prevent inappropriate neonatal discharge of affected babies and subsequent collapse at home. RECURRENCE RISKS OF HLHS The quoted recurrence of HLHS varies from series to series. Nora and Nora suggested a recurrence risk of 2% if a previous child had been affected, based on combined data Ultrasound in Obstetrics and Gynecology Editorial Simpson from Europe and North America between 1966 and 1975. Similar recurrence risks were suggested for coarctation of the aorta and aortic stenosis41. Allan et al. described recurrence of aortic valve atresia in four of 110 cases (3.6%) and recurrence of coarctation of the aorta in four of 61 cases (6.5%) ascertained by fetal echocardiography42. The recurrence risks for congenital heart disease are different if either parent has had congenital heart disease. If the mother has aortic stenosis, then a recurrence risk of 13±18% has been suggested compared to 3% if the father is affected. For coarctation of the aorta a recurrence risk of 4% has been documented if the mother is affected and 2% if the father is affected41. Regardless of exact recurrence risk of these conditions, which may fall into the spectrum of HLHS, there is an increased chance of recurrence above the background population risk. Thus, families who have had an affected child should be offered detailed fetal echocardiography in subsequent pregnancies. For parents, the prospect of a recurrence of congenital heart disease, including HLHS, provokes immense anxiety43. Our strong impression is that parents value early reassurance of normality in subsequent pregnancies. At this author's unit, a tertiary fetal cardiology center, transabdominal fetal echocardiography is offered at 13±14 weeks gestational age to check the main structure of the fetal heart. Echocardiography is repeated on at least one further occasion, later in pregnancy, to exclude more subtle abnormalities and to document the growth of left heart structures. S U M M A RY Hypoplastic left heart syndrome may be accurately diagnosed during fetal life. Prenatal diagnosis provides the opportunity for parents to make an informed choice about their options, including surgery, nonintervention postnatally or termination of pregnancy. Short to medium term survival continues to improve for a condition that was previously invariably lethal. There continues to be a significant mortality and morbidity associated with hypoplastic left heart syndrome, and the long-term prognosis is unknown. Knowledge of the condition prior to birth means that babies who are to undergo surgery present in optimal condition for such interventions. Parents who have had an affected fetus or child should be offered detailed fetal echocardiography to exclude a recurrence in subsequent pregnancies. ACKNOWLEDGEMENTS I am grateful to the organization `Left Heart Matters' for permission to use diagrams from their online handbook of hypoplastic left heart syndrome (www.lhm.org.uk). Department of Fetal Cardiology 15th Floor, Guy's Tower Guy's Hospital St. Thomas' Street London SE1 9RT UK Ultrasound in Obstetrics and Gynecology R E FE R E NC E S 1 Norwood WI, Lang P, Hansen DD Physiologic repair of aortic atresia-hypoplastic left heart syndrome. New England J Med 1983; 308: 23±6 2 Forbess JM, Cook N, Roth SJ, Serraf A, Mayer JE, Jonas RA Tenyear institutional experience with palliative surgery for hypoplastic left heart syndrome. Risk factors related to stage 1 mortality. Circulation 1995; 92 (Suppl.): 262±6 3 Ferencz C, Rubin JD, McCarter RJ, Brenner JI, Neill C, Perry LW, Hepner SI, Downing JW. Congenital heart disease: Prevalence at livebirth: The Baltimore-Washington infant study. Am J Epidem 1985; 121: 31±6 4 Abu-Harb M, Hey E, Wren C. Death in infancy from unrecognised congenital heart disease. Arch Dis Child 1994; 71: 3±7 5 Allan LD, Sharland GK, Milburn A, Lockhart SM, Groves AMM, Anderson RH, Cook AC, Fagg NL. Prospective diagnosis of 1006 consecutive cases of congenital heart disease in the fetus. J Am Coll Cardiol 1994; 23: 1452±8 6 Allan LD, Sharland G, Tynan MJ. The natural history of the hypoplastic left heart syndrome. Int J Cardiol 1989; 25: 341±3. 7 Simpson JM, Sharland GK. Natural history and outcome of aortic stenosis diagnosed prenatally. Heart 1997; 77: 205±10. 8 Maxwell DJ, Allan LD, Tynan M. Balloon aortic valvoplasty in the fetus: a report of two cases. Br Heart J 1991; 65: 256±8. 9 Allan LD, Maxwell DJ, Carminati M, Tynan MJ. Survival after fetal aortic balloon valvoplasty. Ultrasound Obstet Gynecol 1995; 5: 90±1. 10 Sharland GK, Chan K±Y, Allan LD. Coarctation of the aorta: difficulties in prenatal diagnosis. Br Heart J 1994; 71: 70±5. 11 Hornberger LK, Sahn DJ, Kleinman CS, Copel J, Silverman NH. Antenatal diagnosis of coarctation of the aorta: a multicentre experience. J Am Coll Cardiol 1994; 23: 417±23. 12 Hornberger LK, Sanders SP, Rein AJ, Spevak PJ, Parness IA, Colan SD. Left heart obstructive lesions and left ventricular growth in the midtrimester fetus: a longitudinal study. Circulation 1995; 92: 1531±8. 13 Raymond FL, Simpson JM, Sharland GK, Mackie Ogilvie CM. Fetal echocardiography as a predictor of chromosomal abnormality. Lancet 1997; 360: 930 14 Munn MB, Brumfield CG, Lau Y, Colvin EV. Prenatally diagnosed hypoplastic left heart syndrome: outcomes after postnatal surgery. J Maternal-Fetal Med 1999; 8: 147±50 15 Tennstedt C, Chaoui R, Korner H, Dietel M. Spectrum of congenital heart defects and extracardiac malformations associated with chromosomal abnormalities: results of a seven year necropsy study. Heart 1999; 82: 34±9. 16 Natowicz M, Chatten J, Clancy R, Conard K, Glauser T, Huff D, Lin A, Norwood W, Rorke LB, Uri A, Weinberg P, Zackai E, Kelley RI. Genetic disorders and major extracardiac anomalies associated with the hypoplastic left heart syndrome. Pediatrics 1988; 82: 698±706. 17 Allan LD, Apfel HD, Printz BF. Outcome after prenatal diagnosis of the hypoplastic left heart syndrome. Heart 1998; 79: 371±3. 18 Cook AC, Fagg NLK, Sharland GK. Anatomy of the hypoplastic left heart syndrome in the fetus. Cardiol Young 1998; 9 (Suppl.): 2. 19 Bull C (for the British Paediatric Cardiac Association). Current and potential impact of fetal diagnosis on prevalence and spectrum of serious congenital heart disease at term in the UK. Lancet 1999; 354: 1242±7. 20 Rollings S. What next ? The role of the fetal cardiology counsellor following a diagnosis of HLHS. Synergy 1999; 7±8. 21 Chang AC, Huhta JC, Yoon GY, Wood DC, Tulzer G, Cohen A, Mennuti M, Norwood WI. Diagnosis, transport and outcome in fetuses with left ventricular outflow tract obstruction. J Thorac Cardiovasc Surg 1991; 102: 841±8 22 Bove EL. Current status of staged reconstruction for hypoplastic left heart syndrome. Pediatric Cardiol 1998; 19: 308±15 23 Simpson J, Sharland G, Anderson D, Murdoch I, Tynan M. 277 Editorial 24 25 26 27 28 29 30 31 32 33 Hypoplastic left heart syndrome: willingness to initiate treatment is crucial (letter). Br Med J 1997; 314: 1414 Andrews R, Tulloh R, Sharland G, Simpson J, Baker E, Qureshi S, Rosenthal E, Austin C, Anderson D. The Norwood procedure for hypoplastic left heart syndrome: outcome following antenatal and postnatal diagnosis. In Proceedings of the Annual General Meeting of the Royal College of Paediatrics and Child Health, York, April 2000. Ishino K, Stumper O, De Giovanni JJ, Silove ED, Wright JG, Sethia B, de Brawn WJ, Leval M. The modified Norwood procedure for hypoplastic left heart syndrome: early to intermediate results of 120 patients with particular reference to aortic arch repair. J Thorac Cardiovasc Surg 1999; 117: 920±30 Fontan F, Kirklin JW, Fernandez G, Costa F, Naftel DC, Tritto F, Blackstone EH. Outcome after a `perfect' Fontan operation. Circulation 1990; 81: 1520±36 Rogers BT, Msall ME, Buck GM, Lyon NR, Norris MK, Roland JMA, Gingell RL, Cleveland DC, Pieroni DR. Neurodevelopmental outcome of infants with hypoplastic left heart syndrome. J Pediatr 1995; 126: 496±8 Kern JH, Hinton VJ, Nereo NE, Hayes CJ, Gersony WM. Early developmental outcome after the Norwood procedure for hypoplastic left heart syndrome. Pediatrics 1998; 102: 1148±52 Glauser TA, Rorke LB, Weinberg PM, Clancy RR. Congenital brain anomalies associated with the hypoplastic left heart syndrome. Pediatrics 1990; 85: 984±90 Glauser TA, Rorke LB, Weinberg PM, Clancy RR. Acquired neuropathologic lesions associated with the hypoplastic left heart syndrome. Pediatrics 1990; 85: 991±1000 Tworetzky W, McIlhenney DB, Reddy VM, Hanley FL, Silverman NH. Does prenatal diagnosis of hypoplastic left heart syndrome lead to improved surgical outcome? J Am Coll Cardiol 1998; 31 (Suppl.): 71A Starnes VA, Griffin ML, Pitlick PT, Bernstein D, Baum D, Ivens K, Shumway NE. Current approach to hypoplastic left heart syndrome. Palliation, transplantation, or both? J Thorac Cardiovasc Surg 1992; 104: 189±94 Razzouk AJ, Chinnock RE, Gundry SR, Johnston JK, Larsen RL, Baum MF, Mulla NF, Bailey LL. Transplantation as a primary 278 Simpson 34 35 36 37 38 39 40 41 42 43 treatment for hypoplastic left heart syndrome: intermediate-term results. Ann Thoracic Surgery 1996; 62: 1±7 Canter C, Naftel D, Caldwell R, Chinnock R, Pahl E, Frazier E, Kirklin J, Boucek M, Morrow R. Survival and risk factors for death after cardiac transplantation in infants: a multi-institutional study: The Pediatric Heart Transplant Study. Circulation 1997; 96: 227±31 Hehrlein FW, Yamamoto T, Orime Y, Bauer J. Hypoplastic left heart syndrome: `Which is the best operative strategy?' Ann Thorac Cardiovasc Surg 1998; 4: 125±32 Kleinman C. The responsibilities of the perinatal cardiologist: outcomes analysis. Ultrasound Obstet Gynecol 1995; 6: 380±5 Montana E, Khoury MJ, Cragan JD, Sharma S, Dhar P, Fyfe D. Trends and outcomes after prenatal diagnosis of congenital cardiac malformations by fetal echocardiography in a well defined birth population, Atlanta, Georgia. 1990±1994. J Am Coll Cardiol 1996; 28: 1805±9 Buskens E, Grobbee DE, Frohn-Mulder IM, Stewart PA, Juttmann RE, Wladimiroff JW, Hess J. Efficacy of routine fetal ultrasound screening for congenital heart disease in normal pregnancy. Circulation 1996; 94: 67±72 Kumar RK, Newburger JW, Gauvreau K, Kamenir SA, Hornberger LK. Comparison of outcome when hypoplastic left heart syndrome and transposition of the great arteries are diagnosed prenatally versus when diagnosis of these two conditions is made only postnatally. Am J Cardiol 1999; 83: 1649±53 Abu-Harb M, Wyllie J, Hey E, Richmond S, Wren C. Presentation of obstructive left heart malformations in infancy. Arch Dis Child Fetal Neonatal Edition 1994; 71: F179±83 Nora JJ, Nora AH. Update on counselling the family with a firstdegree relative with a congenital heart defect. Am J Med Gen 1988; 29: 137±42 Allan LD, Crawford DC, Chita SK, Anderson RH, Tynan MJ. Familial recurrence of congenital heart disease in a prospective series of mothers referred for fetal echocardiography. Am J Cardiol 1986; 58: 334±7 Rona RJ, Smeeton NC, Beech R, Barnett A, Sharland G. Anxiety and depression in mothers related to severe malformation of the heart of the child and foetus. Acta Paediatrica 1998; 87: 201±5 Ultrasound in Obstetrics and Gynecology