* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download DHFR catalyzes the transfer of a hydride from NADPH to
Survey
Document related concepts
Nutriepigenomics wikipedia , lookup
Designer baby wikipedia , lookup
Expanded genetic code wikipedia , lookup
Neocentromere wikipedia , lookup
Oncogenomics wikipedia , lookup
Flavin adenine dinucleotide wikipedia , lookup
Microevolution wikipedia , lookup
Point mutation wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Genome (book) wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Transcript
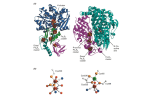
Dihydrofolate reductase, (DHFR) Dihydrofolate reductase, or DHFR, is an enzyme that reduces dihydrofolic acid to tetrahydrofolic acid, using NADPH as electron donor, which can be converted to the kinds of tetrahydrofolate cofactors used in 1-carbon transfer chemistry. In humans, the DHFR enzyme is encoded by the DHFR gene. It is found in the q11→q22 region of chromosome 5. Bacterial species possesses distinct DHFR enzymes (based on their pattern of binding diaminoheterocyclic molecules), but mammalian DHFRs are highly similar.[4] The plasmidencoded DHFR (R67 dihydrofolate reductase) shows a high level of resistance to the antibiotic trimethoprim. It is a homotetramer with an unusual pore, which contains the active site, passing through the middle of the molecule. Its structure is unrelated to that of chromosomal DHFRs. A central eight-stranded beta-pleated sheet makes up the main feature of the polypeptide backbone folding of DHFR. Seven of these strands are parallel and the eighth runs antiparallel. Four alpha helices connect successive beta strands. Residues 9 – 24 are termed “Met20” or “loop 1” and, along with other loops, are part of the major subdomain that surround the active site.[8] The active site is situated in the N-terminal half of the sequence, which includes a conserved Pro-Trp dipeptide; the tryptophan has been shown to be involved in the binding of substrate by the enzyme. Function Dihydrofolate reductase converts dihydrofolate into tetrahydrofolate, a methyl group shuttle required for the de novo synthesis of purines, thymidylic acid, and certain amino acids. While the functional dihydrofolate reductase gene has been mapped to chromosome 5, multiple intronless processed pseudogenes or dihydrofolate reductase-like genes have been identified on separate chromosomes Mechanism DHFR catalyzes the transfer of a hydride from NADPH to dihydrofolate with an accompanying protonation to produce tetrahydrofolate. In the end, dihydrofolate is reduced to tetrahydrofolate and NADPH is oxidized to NADP+. The high flexibility of Met20 and other loops near the active site play a role in promoting the release of the product, tetrahydrofolate. In particular the Met20 loop helps stabilize the nicotinamide ring of the NADPH to promote the transfer of the hydride from NADPH to dihydrofolate. Since folate is needed by rapidly dividing cells to make thymine, this effect may be used to therapeutic advantage. DHFR can be targeted in the treatment of cancer. DHFR is responsible for the levels of tetrahydrofolate in a cell, and the inhibition of DHFR can limit the growth and proliferation of cells that are characteristic of cancer. Methotrexate, a competitive inhibitor of DHFR, is one such anticancer drug that inhibits DHFR. Other drugs include trimethoprim and pyrimethamine. These three are widely used as antitumor and antimicrobial agents.




![1,2-DIHYDROPYRAZOLO[1,2-A]](http://s1.studyres.com/store/data/002555217_1-0c89b3c15753508fdc505e732fbfe183-150x150.png)