* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The role of Pax-1 in axial skeleton development
Survey
Document related concepts
Gene expression programming wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Point mutation wikipedia , lookup
Gene expression profiling wikipedia , lookup
Designer baby wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Transcript
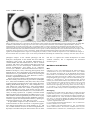
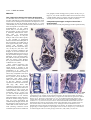
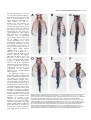
1109 Development 120, 1109-1121 (1994) Printed in Great Britain © The Company of Biologists Limited 1994 The role of Pax-1 in axial skeleton development Johan Wallin1, Jörg Wilting2, Haruhiko Koseki1, Rüdiger Fritsch3, Bodo Christ2 and Rudi Balling1,4,* 1Department of Developmental Biology, Max-Planck Institute of Immunobiology, 2Department of Anatomy, University of Freiburg 3Max-Planck Institute for Biophysical Chemistry, Göttingen 4Institut für Säugetiergenetik, GSF-Forschungszentrum Neuherberg, FRG Stübeweg 51, D-79108 Freiburg, FRG *Author for correspondence at address4 SUMMARY Previous studies have identified a single amino-acid substitution in the transcriptional regulator Pax-1 as the cause of the mouse skeletal mutant undulated (un). To evaluate the role of Pax-1 in the formation of the axial skeleton we have studied Pax-1 protein expression in early sclerotome cells and during subsequent embryonic development, and we have characterized the phenotype of three different Pax-1 mouse mutants, un, undulated-extensive (unex) and Undulated short-tail (Uns). In the Uns mutation the whole Pax-1 locus is deleted, resulting in the complete absence of Pax-1 protein in these mice. The other two genotypes are interpreted as hypomorphs. We conclude that Pax-1 is necessary for normal vertebral column formation along the entire axis, although the severity of the phenotype is strongest in the lumbar region and the tail. Pax-1-deficient mice lack vertebral bodies and intervertebral discs. The proximal part of the ribs and the rib homologues are also missing or severely malformed, whereas neural arches are nearly normal. Pax-1 is thus required for the development of the ventral parts of vertebrae. Embryonic analyses reveal that although sclerotomes are formed in mutant embryos, abnormalities can be detected from day 10.5 p.c onwards. The phenotypic analyses also suggest that the notochord still influences vertebral body formation some days after the sclerotomes are formed. Furthermore, the notochord diameter is larger in mutant embryos from day 12 p.c., due to increased cell proliferation. In the strongly affected genotypes the notochord persists as a rod-like structure and the nucleus pulposus is never properly formed. Since the notochord is Pax-1-negative these findings suggest a bidirectional interaction between notochord and paraxial mesoderm. The availability of these Pax-1 mutant alleles permitted us to define an early role for Pax-1 in sclerotome patterning as well as a late role in intervertebral disc development. Our observations suggest that Pax-1 function is required for essential steps in ventral sclerotome differentiation, i.e. for the transition from the mesenchymal stage to the onset of chondrogenesis. INTRODUCTION gives rise to vertebral bodies and intervertebral discs whereas the caudal halves of the paired lateral sclerotome areas are the origins of neural arches, pedicles and ribs (Verbout, 1985; Christ and Wilting, 1992). The axial regions of highest cell density, the intervertebral disc anlagen, are positioned approximately midsegmentally with respect to the former somite boundaries. By cell labelling experiments it has been shown that one vertebral body is derived from cells originating from two adjacent somites (Bagnall, 1992; Ewan and Everett, 1992). The mechanisms that control these morphological events in axial and lateral regions have not been studied, however. The existence of a large number of mouse mutants with specific malformations in vertebral column development (Grüneberg, 1963; Theiler, 1988) is an important resource for investigating the molecular and morphogenetic events that lead to the development of this structure. One of these mutants, undulated (un; Wright, 1947), carries a point mutation in the Pax-1 gene (Balling et al., 1988), which is transcriptionally activated in sclerotome cells at the time of differentiation of the epithelial somites (Deutsch et al., 1988). A detailed mor- The vertebral column is derived entirely from cells in the ventral halves of the somites. In morphological terms the events leading to axial skeleton formation have been extensively studied in several species, and are well described (Verbout, 1985; Christ and Wilting, 1992). The vertebral column is the most conspicuously segmented structure of the vertebrate body. The metameric pattern is initially laid down during the formation of somites, yet there is not a clear relationship between the vertebral units and the initial somites. Upon somite differentiation the ventral parts de-epithelialize and form the mesenchymal sclerotomes, which give rise to precartilaginous structures as well as connective tissue. This process is dependent on signals from the notochord (Watterson et al., 1954; Pourquie et al., 1993). Some sclerotome cells migrate medially and form the perichordal tube, which is initially unsegmented and uniform in density. Thereafter a segmented pattern of condensations starts to appear, the lateral regions slightly preceding the axial ones. The perichordal tube Key words: Pax-1, vertebral column, sclerotome, notochord, mouse mutants 1110 J. Wallin and others Fig. 1. Analysis of Pax-1 expression in the developing vertebral column. (A) Whole-mount in situ hybridization of a day 10.5 wild-type embryo, showing strong Pax-1 expression in the sclerotome. In each segment, the caudal half displays stronger expression than the cranial half. In addition, a thin domain of strong expression can be seen in the top of the cranial half. Arrows indicate segment boundaries. (B) Pax-1 immunostaining of a frontal section from a day 10.5 wild-type embryo. Cranial is towards the top and lateral to the left of the picture. The notochord (n) can be seen on the right-hand side and the spinal nerve (sn) in the cranial part of each segment. Most sclerotome (s) cells, seen between the dermomyotome (dm) and the notochord, are Pax-1-positive, whereas dermomyotome, nerve and notochord cells are negative. The localization of the spinal nerve as well as cell density, might explain the banded staining pattern in the whole-mount staining in A. (C) A sagittal section through the thoracic region of a wild-type day-14.5 embryo; ventral is to the left. Expression is now confined to the anlagen of the intervertebral discs (ivd) and to a layer of cells in the perichondrium surrounding the vertebral body (vb) anlage. Scale bars: (B,C) 100 µm. phological analysis of the skeletal phenotype and the embryonic development of this mutant has been made by Grüneberg (1950, 1954). He described malformations of vertebral bodies and intervertebral discs as well as vertebral processes, and came to the conclusion that these result from decreased sizes of the mesenchymal condensations that precede chondrification. Two alleles of un, undulatedextensive (unex; Wallace, 1985) and Undulated short-tail (Uns; Blandova and Egorov, 1975), both being phenotypically more affected than un, have subsequently been isolated. Both these mutants have Pax-1 gene deletions, the latter one removing the entire Pax-1 locus (Balling et al., 1992; Dietrich S., Gruss P., and R.B., unpublished observations). The Pax genes form a family of developmental control genes that have recently been the focus of great interest (Chalepakis et al., 1992; Gruss and Walther, 1992; Noll, 1993). They encode sequence-specific transcription factors that contain the DNAbinding paired-domain (Bopp et al., 1986; Chalepakis et al., 1991; Adams et al., 1992; Zannini et al., 1992), and in addition some of the Pax proteins also contain a paired-type homeodomain (Walther et al., 1991). In the mouse, nine Pax genes have been identified to date (Walther et al., 1991; Wallin et al., 1993), all being expressed in a spatially restricted manner beginning during early organogenesis. In addition to Pax-1, two other members of the Pax gene family have been shown to play important roles in mouse embryogenesis. Mutations in the Pax3 and Pax-6 genes cause the Splotch and Small-eye phenotypes, respectively (Epstein et al., 1991; Hill et al., 1991). To gain further insight into early mechanisms of vertebral column formation we have carried out a comparative phenotypic analysis of the three Pax-1 mouse mutants, un, unex and Uns, of which the latter two have not been investigated before. By analysis of mutant embryonic and new-born mice we show that Pax-1 is required for normal development of ventral vertebral structures, but is dispensable for sclerotome formation per se. MATERIALS AND METHODS Mice undulated (un) mice were purchased from the Jackson laboratory. Undulated-short tail (Uns) and undulated-extensive (unex) mutant mice were kindly provided by Dr A. M. Malashenko, Krosnogorsk, Russia and Dr J. L. Cruickshank, Leeds, England, respectively. These mutants were backcrossed onto the C57BL/6 strain. On this genetic background homozygous un and unex mice show reduced fertility, particularly unex. Homozygous unex mice are often born small, in which case they also remain smaller and die earlier than their littermates. N1 to N5 generations were used for analysis. Embryos were recovered on days 9.5-18.5 p.c. where day 0.5 was 12 a.m. on the day of detection of the vaginal plug. On average, litters were born on day 19 p.c. As controls, +/+ or un/+ littermates were normally used. In exceptional cases C57BL/6 embryos of the corresponding stages were used. Genotyping Genotypings were made either by PCR analysis on embryonic DNA prepared from yolk-sacs or by Southern blotting of placental DNA. Genomic Southern blots were performed according to standard procedures (Sambrook et al., 1989). For PCRs two different primer pairs were used: (1) with primers flanking the mutated HaeIII restriction site in un and (2) specific for the fifth exon deleted in unex. These Pax1-specific oligonucleotides were: (1) 5′-CAGAGCAGACGTACGGCGAAG-3′ and 5′-AGGCAAAGATGCCAGGATCCC-3′ and for (2) 5′-AGAGCCATCAGCATGGTTTCG-3′ and 5′-TGGAGGGAGTCCAGATTAAGC-3′. PCR reaction conditions were: 30 cycles of denaturation for 1 minute at 94°C, reannealing for 1 minute at 60°C Pax-1 and axial skeleton development 1111 Fig. 2. A schematic representation of the molecular basis of the Pax-1 deficiency of the un and Uns alleles (A), and the external appearance of adult homozygous un (B), homozygous unex (C) and heterozygous Uns mice (D). In the upper part of A, the exon-intron organization of the Pax-1 gene is depicted (Deutsch, 1990) The coding region is indicated as filled boxes and the paired box is hatched. The position of the point mutation in un is marked by a cross. The exact locations of the deletion breakpoints in Uns have not been determined. and extension for 1 minute at 72°C for 1 minute; 2.5 units Taq polymerase (Amersham) using assay conditions specified by the manufacturer in a 50 µl reaction volume. PCR products were separated in agarose gels and visualized by ethidium bromide staining. For typing of un, PCR products were digested with HaeIII before gel separation, producing allele-specific band sizes that could easily be scored. For diagnosis of Uns and unex homozygotes, a primer pair specific for an unlinked gene (Pax-9) was included as an internal control. A large number of un and unex embryos were also obtained from crosses of homozygous mice. Histology For histological analysis, day 9.5-18.5 embryos were fixed in Bouin’s solution and embedded in paraffin. 8 µm sections were stained either in haematoxylin-eosin or Azan. In addition, for day 10.5-12.5 p.c. embryos of the Uns/Uns, Uns/un and +/+ genotypes, semi-thin sections were made, focusing on the strongly affected lumbar region. Embryos were fixed in Karnovsky’s solution, embedded in plastic and 0.75 µm sections were stained with toluidine blue. Skeletal preparations Skeletal preparations were made by a slight modification of the Alcian blue/alizarin red staining procedure described by Kessel et al. (1990). Specimens were fixed in 99% ethanol for 24 hours (fetuses older than day 15 p.c. were first deskinned and eviscerated), and then kept in acetone for another 24 hours. Incubation in staining solution (1 vol. of 0.3% Alcian blue in 70% ethanol, 1 vol. of 0.1% alizarin red S in 96% ethanol, 1 volume of absolute acetic acid, and 17 volumes of 70% ethanol) was performed for 4-6 hours at 37°C and then overnight at room temperature. Samples were rinsed in water and kept in 1% potassium hydroxide/20% glycerol at 37°C over-night, with additional incubation at room temperature until complete clearing. For long term storage, specimens were transferred into 50%, 80% and finally 100% glycerol. In situ hybridization Pax-1 riboprobes were generated from a HincII-SacI paired box fragment using either 35S-UTP or digoxigenin-11-UTP labelling for in situ hybridization on sections or in whole embryos, respectively. Fixation, hybridization and subsequent detection procedures were essentially as described by Kessel and Gruss (1991) and Rosen and Beddington (1993). BrdU labelling Cell proliferation was monitored by incorporation of BrdU into embryos in utero. Pregnant mice were injected intraperitoneally with a 5 mg/ml BrdU (Sigma) solution in PBS. The amount injected was approximately 50 µg/g body weight. After 2 hours the mice were killed and embryos isolated, fixed and embedded in paraffin. 8 µm sections were stained with an anti-BrdU antibody as described below and counterstained with basic fuchsin or hematoxylin. The number of labelled cells as well as total cell numbers were scored. Immunohistochemistry For immunostainings, embryos were fixed in 3% acetic acid in absolute ethanol at 4°C over-night, incubated first in absolute ethanol and then in xylene, both twice for 30 minutes, followed by a 1:1 mixture of xylene:paraffin for 30 minutes at 55°C, infiltrated with paraffin by three incubations for 1 hour at 55°C and embedded. The generation of the Pax-1-specific antiserum has been previously described (Chalepakis et al., 1991). The mouse monoclonal anti-BrdU antibody was purchased from Dako. For BrdU immunocytochemistry, sections were deparaffinized and air-dried over-night. They were then incubated in 2 N HCl for 30 minutes, washed in PBS, pH 6.0, for 1 minute and in PBS, pH 7.4 (hereafter called PBS) for 5 minutes. Primary antibody was diluted 1:50 in PBS, 1% BSA, 0.25% Tween-20 and incubated for 1 hour. Following washes, detection was made with the biotin-streptavidinperoxidase amplification procedure, UniTect ABC-kit (Dianova). Pax-1 immunostaining was made on deparaffinized sections that were first bleached in 0.3% H2O2 in methanol for 30 minutes, washed in PBS, blocked with 10% normal goat serum, in PBS for 60 minutes and then incubated with the antiserum diluted 1:200 in the blocking solution. Detection was made with peroxidase-conjugated goat-antirabbit IgG (Sigma), and diaminobenzidine was used as chromogen. 1112 J. Wallin and others RESULTS root ganglia) remain strongly Pax-1-positive at day 14.5 p.c. (Figs 1C, 7G). In the vertebral column of new-born mice only a small number of Pax-1-positive cells can be observed in the ventral part of the annulus fibrosus (data not shown). Pax-1 expression during sclerotome development To determine the role of Pax-1 in sclerotome differentiation, we have analysed Pax-1 RNA and protein expression in early Comparative phenotypic analysis of three Pax-1 somites and in subsequent axial skeleton development. Pax-1 mutant alleles transcription can first be detected around day 8.5 p.c. in the ventromedial part of newly formed somites (data not shown). We have chosen to study the phenotype of three spontaneously This corresponds to the time of deepithelialization of the ventral somite half, i.e. the emergence of the sclerotome. At first, expression is uniformly distributed in sclerotome cells. In whole-mount expression analysis of day-10.5 embryos with a Pax-1 RNA probe, it was possible to distinguish between cranial and caudal parts of the sclerotomes, the caudal part of each segment being more strongly positive and extending more dorsally than the cranial one (Fig. 1A). At day 12.5, the Pax-1 transcripts are mainly confined to the anlagen of the intervertebral disc and weaker domains can be seen in the perichondria lining the cartilage blastemas of the vertebral bodies, the pedicles and the proximal ribs (data not shown). These findings are in good accordance with those of Deutsch et al. (1988). Pax-1 protein expression was detected with a rabbit antiserum raised against a peptide in the carboxy-terminal end of the protein (Chalepakis et al., 1991). The protein is expressed with a delay of about 1 day compared to the onset of transcription. First, at day 9.5, Pax-1 protein can be detected in sclerotome cells, albeit weakly (data not shown). Immunostaining of day-10.5 embryos revealed expression in almost all sclerotome cells (Fig. 1B). These cells continue to express Pax-1 up to the time when mesenchymal cells start to chondrify in order to form the vertebral bodies, which takes place around day 12.0 p.c. At day 12.5 p.c., the cells of the anlagen of the Fig. 3. Histological analysis at day 18.5 p.c. Sagittal sections of +/+ (A) and Uns/Uns (B). Note the vertebral bodies are not stained, complete absence of vertebral column structures adjacent to the aorta in the mutant (arrowhead). of the internal organs and a reduced while the anlagen of the interverte- The shortening of the vertebral column leads tos a compression s bral discs remain strongly positive thoracic cavity. The airways of the lungs in Un /Un are not dilated and the lung appears more litter-mate. Arrows indicate lungs. Higher magnification of thoracic (Fig. 7E). Cells surrounding the compact than in the wild-type vertebrae of +/+ (C) and Uns/Uns (D) are shown. The skeletal elements in the mutant section are vertebral body anlagen, those in the much thinner dorsoventrally and resemble ventral arches rather than proper vertebral bodies (vb). disc anlagen as well as those in the Note also the absence of intervertebral discs (ivd). Although consecutive boundaries can be cranial half of each lateral sclero- identified, the nucleus pulposus and annulus fibrosus are missing. (E) Example of fused dorsal root tome (forming connective tissue ganglia (drg) over three segments in a parasagittal section of Uns/Uns at day 18.5 p.c. Such fusions around the spinal nerves and dorsal are found along the entire axis in this genotype. Scale bars: (A,B) 2 mm, (C-E) 200 µm. Pax-1 and axial skeleton development 1113 arisen mouse mutants, un, unex and Uns, since the molecular characterization of these mice has shown that there is a point mutation in the paired domain of un (Balling et al., 1988), whereas unex and Uns display Pax-1 gene deletions. In Uns the entire locus is deleted (schematically depicted in Fig. 2A). The absence of the Pax-1 gene in this mutant has previously been demonstrated for the paired box region (Wallin et al., 1993), and has been extended to the 5′-flanking sequences and 3′-untranslated region (data not shown). The size of the Uns deletion has not been exactly determined, but a major chromosomal deletion has been excluded by karyotyping and by demonstration of the presence, in Uns homozygous DNA, of several closely linked DNA markers (GBASE, March, 1993, A. Y. Hillyard, D. P. Doolittle, M. T. Davisson, and T. H. Roderick, The Jackson Laboratory, Bar Harbor, ME) (data not shown). In unex the fifth exon, which includes part of the carboxy-terminal coding sequence, is deleted (Dietrich S., Gruss P., and R. B., unpublished observations). The allelic nature of all three mutations has also been confirmed by inter-crosses. In no combination of the different mutants was a complementation effect observed. The phenotypic appearance of adult mutant mice is characterized by their shortened and kinky tails (Fig. 2B-D). un is known as a recessive mutation (Wright, 1947), but in our colony we have observed a small percentage of heterozygous animals that have slight but significant distal tail kinks, which is also true for unex (data not shown). Thus although un/+ embryos occasionally do display very mild abnormalities, they serve as good controls for the stronger genotypes for all practical purposes. Uns is invariably semidominant and produces a clear phenotype in the heterozygous situation. Uns/Uns mice die shortly after birth. Their tails are only rudimentary and the trunks considerably shorter than those of wild-type littermates (Fig. 3A,B), nevertheless pups show normal movements at Fig. 4. Comparison of the vertebral column phenotypes at the new-born stage. Whole-mount skeletal preparations of the following genotypes are shown: +/+ (A), un/un (B), unex/unex (C), Uns/+ (D), Uns/un (E) and Uns/Uns (F). Note the phenotypic similarity between the un/un and unex/unex skeletons. The strongest abnormalities can be seen in the lumbar region where split vertebrae and dual ossification centers as well as affected transverse processes are found. In Uns/+ (D), the ossification centers are slightly smaller and the lateral sclerotome derivatives, transverse processes and ribs, are clearly affected. Note for example the floating 13th rib pair. In Uns/un (E), ventral vertebral structures are severely affected in the cervical and lower thoracic-lumbar region. The last four rib pairs are floating. The most extreme phenotype, that of Uns/Uns (F), is an accentuation of what is seen in Uns/un. Affected structures are now further decreased in size, displaying a complete lack of ventral skeletal structures in lower thoracic-lumbar region. Arrows point out abnormal proximal ribs. 1114 J. Wallin and others Pax-1 and axial skeleton development 1115 Fig. 6. Alcian blue stainings of fetal skeletons. Dorsal view of day 13.5 p.c. +/+ (A) and Uns/Uns (B) specimens, and detail of a day 14.5 p.c. unex/unex skeleton (C). In the cervical and lower thoracic-lumbar region the cartilage precursors of the vertebral bodies are absent (B). The change in phenotypic pattern between the sacral and caudal regions can also be seen. Cartilage structures surrounding the notochord can be seen in the midline of the tail, while there is an absence of pedicle anlagen bilaterally. (C) The vertebral body formation is significantly more advanced at the side of the notochord, which has been laterally displaced (arrow). birth. The reason for the postnatal lethality, which is also observed for the Uns/un and Uns/unex compound heterozygotes, could not be exactly determined. The most likely explanation is respiratory failure as a result of lung space constraints. Histological sections of Uns/Uns at day 18.5 p.c. show lungs with drastically reduced airway lumina (Fig. 3A,B). Fig. 5. Analysis of separate skeletal elements at the new-born stage. Frontal views of the lower thoracic-upper lumbar region of the +/+, un/un, Uns/+, Uns/un and Uns/Uns genotypes (A-E). In the new-born wild-type skeleton (A) the ribs form joints at the lateral aspects of the intervertebral discs, which at this stage are well-defined structures. In un/un, however, the 13th rib pair fuses to the vertebral body (arrowhead) and the discs are virtually absent (arrow). In Uns/+ (C), the annulus fibrosus is thinner than in the wild-type (arrow), whereas Uns/un (D) and Uns/Uns (E) have no bodies and discs. Note also the notochord remnant in Uns/un (D) which is covered by a cartilage layer. The ninth thoracic vertebrae of the same genotypes as A-E are compared in F-J, respectively. The principal similarity of the phenotypes can clearly be seen; vertebral bodies are reduced or absent and the proximal part of the rib is abnormal or missing. A comparison of isolated vertebrae from different craniocaudal levels of +/+ (K-O) and Uns/Uns (P-T) new-born mice are also shown. Atlas (K,P), sixth cervical vertebrae (L,Q), fourth lumbar vertebrae (M,R), second sacral (N,S) and tails (O,T). The +/+ tail specimen in O shows the last sacral and the first three caudal vertebrae, while the Uns/Uns (T) tail is composed of the last two sacral vertebrae as well as remnants of several caudal vertebrae. Arrows indicate the position of the first caudal vertebrae. With the exception of the atlas and the tail, the vertebral abnormalities at different levels are principally similar, i.e. lack of ventral and ventrolateral elements. A slight effect on of the neural arches can also be noted as a reduction of the spineous processes. The dens axis is fused to the atlas in Uns/Uns (arrowhead; P). The tails are viewed at a slight angle and the abnormal tail of Uns/Uns has a trifurcated appearance (T). One ventral and two lateral structures are observed. These vertebrae remnants are fused along the craniocaudal axis. ivd (intervertebral disc), vb (vertebral body), n (notochord), L1 (first lumbar vertebra), T (thoracic), C (cervical), S (sacral), vt (ventral tubercle), tp (transverse process). Whereas the kinks in un/un are soft and can easily be corrected upon pressure, the tail abnormalities in unex/unex and Uns/+ are more pronounced and rigid, partially due to vertebral fusions. The increase in severity of the phenotypes, can be interpreted as a gradual loss of Pax-1 function in the different mutants. In general, the axial skeleton abnormalities in these mutants are qualitatively similar but quantitatively different. This is more apparent when a detailed analysis of the vertebral components is made. As the strongest phenotypes result in postnatal death, we have concentrated our analyses and comparisons on embryos up to the new-born stage. Abnormalities in the vertebral column of new-born mice All undulated mutants display phenotypic abnormalities of the vertebral column along the entire axis. A list of typical vertebral column aberrations is given in Table 1. The abnormalities affecting vertebral bodies or other axial skeletal elements are similar in all the mutants, but can be found to different degrees; the Uns/Uns genotype being the most strongly affected. The severity of defects is not evenly distributed along the axis and is most pronounced in the lumbar Table 1. Types of skeletal abnormalities that are observed in the vertebral column of un, unex and Uns mice Perichordal tube derivatives Absence of vertebral body Split vertebral body “Ventral arches” Fusions between atlas and dens axis Reduced and malformed vertebral body (dual ossification centers) Absent or reduced intervertebral discs “Haemal arches” (in proximal part of tail) Fusions between vertebral bodies (mainly seen in tail) Lateral sclerotome derivatives Absent or reduced transverse processes Absent or reduced ribs Fusions between ribs and vertebral bodies Fusions between adjacent neural arches Reduced spinous processes Absent pedicles (only in caudal vertebrae) 1116 J. Wallin and others region and the tail. A comparison of skeletal preparations at the new-born stage is shown in Fig. 4. In the wild type, single ossification centres are formed in all vertebral bodies (Fig. 4A). The un and unex homozygous new-borns show split vertebrae, vertebrae with dual ossification centers and missing transverse processes primarily in the lumbar region (Fig. 4B,C). These phenotypes are very similar, although unex has slightly more severe malformations and a few more segments are affected. In the case of Uns a mild phenotype can already be seen in heterozygous mice (Fig. 4D). Vertebral body ossification centers are smaller than normal and transverse processes as well as the proximal parts of ribs are missing or reduced. Uns/Uns mice, with a total Pax-1 deficiency, completely lack vertebral bodies in the lower thoracic and lumbar regions (Fig. 4F). The compound heterozygote Uns/un is intermediate in phenotype between Uns/Uns and un/un mice (Fig. 4E). On closer inspection of the lower thoracic and upper lumbar regions, it is evident, that with the exception of Uns/+, the different mutants lack intervertebral discs (Figs 3C,D and 5AE). In Uns/+ the discs are thinner in the periphery, indicating a disturbance in the formation of the annulus fibrosus. The intervertebral disc is, at the new-born stage, composed partly of hyaline cartilage, and can therefore be stained with Alcian blue. In adult mice the hyaline cartilage has been replaced by fibrous cartilage (Theiler, 1988) and appears clear in wholemount skeletal preparations. In adult skeletal preparations the lack of discs in undulated could be confirmed (data not shown). In extreme cases the lack of discs leads to vertebral body fusions. On rare occasions mice of the Uns/unex genotype have survived after birth and at most lived up to five months of age. The vertebral column of a few of these mice displayed multiple fusions (data not shown). Thus, in all mutants we detect disturbed formation of both vertebral bodies and intervertebral discs. Furthermore, in all cases the proximal parts of the ribs as well as the rib homologues, the transverse processes, are affected. In un and unex the last pairs of ribs are shorter and fuse abnormally to the vertebral bodies. Uns/+, Uns/un and Uns/Uns display more severe abnormalities, with several floating rib pairs and the 13th pair is completely missing in Uns/Uns (Fig. 5A-E). A systematic comparison of the +/+, un/un, Uns/+, Uns/un and Uns/Uns genotypes at the level of the ninth thoracic vertebra clearly shows that the basic phenotype is essentially the same, and varies only in degree of severity between the alleles (Fig. 5F-J). Although we observe small variations in phenotypic severity within one allele (none of the strains are completely inbred), these are insignificant compared to the variation between alleles. Comparison of single vertebrae was made by dissection of whole-mount skeletal preparations. In Fig. 5K-T representative vertebrae from cervical, lumbar, sacral and caudal regions from wild-type and Uns/Uns mice are shown. From this analysis it is evident that vertebral body and rib/transverse process formation are abnormal at all levels. Where a ventral ossified structure can be seen, it resembles a pair of ventrally fused neural arches rather than a vertebral body (Fig. 5J,S). Therefore, Uns/Uns mice are deficient in structures derived both from the perichordal tube and the ventralmost parts of lateral sclerotomes. The neural arches are fairly normally shaped. Reductions of the spinous and articular processes as well as rare fusions between adjacent neural arches can be detected. These deformities are discrete in comparison to the ventral ones and may to a large extent be due to the compression of the vertebral column. The tail vertebrae display a phenotype which is different from the rest of axis. The tailremnant in Uns/Uns gives a trifurcated appearance, representing vestigial vertebral bodies and paired neural arches (Fig. 5T). The pedicles, the structures connecting bodies and neural arches, are lost. These malformed structures display only scant signs of segmentation and are fused to the tip of the tail. First phenotypic sclerotome abnormality at day 10.5 p.c. of development To study when the Pax-1 protein starts to exert its effect we have conducted a detailed morphological analysis of mutant embryos. We have concentrated our study primarily on the lumbar region in the different mutants with particular emphasis on the strongest genotypes Uns/un and Uns/Uns. The compound heterozygote Uns/un has the advantage that the Pax-1 RNA and protein expressed from the un allele can be detected in the mutant embryos, although functionally this mutant is almost as affected as the null-allele Uns/Uns. Analyses of cartilage formation in skeletal clearance preparations, revealed that axial structures in the vicinity of the notochord never chondrify in mutant embryos (Fig. 6B). The mutant phenotype is visible from day 11.5 p.c., the first timepoint when Alcian blue can be used to stain the developing skeleton. Therefore, in agreement with the earlier results of Grüneberg (Grüneberg, 1954), it can be concluded that the malformations are formed at an early embryonic stage and that the primary function of Pax-1 should be looked for before chondrogenesis commences. This lack of axial chondrogenesis most often leads to a lateral displacement of the notochord. Vertebral body formation is always more normal on the side of the notochord. This phenomenon, best observed in unex/unex skeletal preparations (Fig. 6C), suggests that vertebral body formation is also dependent on a late notochord function. Histological analysis of early vertebral column formation, reveals that sclerotome formation occurs largely normally in the Pax-1-deficient mice. Morphological abnormalities can first be detected at day 10.5 p.c., when the lumbar sclerotome region already is abnormal compared with controls. In the wild type, densely and loosely arranged areas are present and the axial cells are oriented towards the notochord, whereas in the most affected mutants the sclerotome cells are evenly distributed (Fig. 7A,B). Abnormalities in the perichordal tube and lateral sclerotome regions At day 12.5 p.c. more profound changes can be detected. In mid-sagittal sections of Uns/Uns and Uns/un embryos, in the region of the perichordal tube, cell numbers are strongly reduced, corresponding to the loss of the anlagen for the vertebral bodies and intervertebral discs (Fig. 7E,F). The few cells present in the perichordal tube of Uns/un embryos at this stage do not display the normal metameric arrangement of condensed intervertebral disc anlagen and cell-sparse vertebral body anlagen. We performed immunostainings on mutant and wild-type embryos to compare the distribution of Pax-1expressing cells. As can be seen in Fig. 7, the Pax-1-positive domains are much wider in the craniocaudal dimension in the Uns/un mutant. The observation that most cells are Pax-1- Pax-1 and axial skeleton development 1117 Fig. 7. Histological (A-D) and immunohistochemical (E-H) analysis of mutant and wild-type embryos. Sclerotome (s) is formed but displays abnormal organization in transverse semi-thin sections of day 10.5 +/+ (A) and Uns/un (B) embryos. In the wild-type embryo a clear mediolateral organization can be observed, with a cell-dense region next to the dermomyotome (dm) and fewer cells surrounding the notochord. In the mutant, however, the mesenchymal cells are disorganized and very few cells can be observed in the perichordal space. Arrowheads indicate the position of the notochord. Frontal sections of day 12.5 +/+ (C) and Uns/un (D) embryos through the lumbar-sacral region. Note the reduced size of the cell condensations that surround the notochord (n). Also the lateral sclerotome regions are affected. Loss of caudal (ca) halves as well as over-abundance of cells surrounding the spinal nerves in the cranial (cr) halves are observed. Arrowheads indicate the position of segment boundaries. In Pax-1 immunostainings of sagittally sectioned day 12.5 un/+ (E) and Uns/un (F) embryos, the reduction of perichordal tube size can be clearly observed. Note also the absence of well-defined segmentation. Parasagittal sections of sacral regions in day 14.5 un/+ (G) and Uns/un (H) embryos show the absence of ventrolateral skeletal structures, i.e. transverse processes (tp), are observed in the mutant. Instead, spinal nerves are surrounded by Pax-1-positive cells only. nt (neural tube), na (neural arch). Scale bars: (A-D,G,H) 100 µm, (E,F) 250 µm. positive reflects the lack of chondrogenesis, which normally coincides with loss of Pax-1 expression as described previously. In Uns/Uns embryos, a more severe loss of cells in the perichordal tube could be observed and the total absence of Pax-1 protein could be confirmed (data not shown). To analyse axial and lateral relationships, frontal sections of mutant embryos were made at day 10.5-12.5 p.c. In sections of the ventral part of the developing vertebral column in day12.5 p.c. mutant embryos, a profoundly altered organization of the lateral lumbosacral sclerotome domains, in addition to the poorly developed perichordal tube, was revealed. Instead of the normal division in cranial and caudal halves, only one ‘compartment’ with equal, fairly high, cell density is detected. The spinal nerve is found in the middle, surrounded by concentric layers of fairly dense cells (Fig. 7C,D). These cells are all staining positive for Pax-1 (Fig. 7G,H). The effect is an expansion of mesenchymal regions at the expense of cartilage formation. Lack of the caudal condensations in these ventrolateral regions later results in the lack of transverse processes and proximal rib structures in the vertebral column of newborn mice. In more dorsal sections, through the anlagen of the neural arches, the craniocaudal division of each segment appears more normal. Thus, Pax-1 mutants do not have difficulty in establishing craniocaudal polarity per se, but rather display a loss of such polarity in the ventral domain of the sclerotome due to the loss of skeletal precursor structures. The notochord and dorsal root ganglia do not develop normally in undulated mice When analysing sclerotome morphology in mutant embryos, we noticed that the notochord appeared larger than in wild-type controls at day 12.5-13.5 p.c. Transverse sections reveal more cells per section and a changed notochordal sheath in the mutants (Fig. 8). Consistent with this observation we found a dramatic increase in the rate of cell proliferation at the corresponding stage. In mutant embryonic notochord, a seven to tenfold increase in the number of BrdU-labelled nuclei was detected, at day 12.5 p.c. (Fig. 8E,F and Table 2). The finding 1118 J. Wallin and others Table 2. Determination of notochordal cell proliferation rates at day 12.5 p.c. +/+ un/+ Percentage of labelled cells 1.8 2.5 Average number of labelled cells/section 0.45 0.45 Uns/un 17 3.9 Uns/Uns 22 6.4 Replicating embryonic cells were labelled with BrdU in utero. For each genotype 18 transverse sections from the lumbar region of two separate embryos have been analysed. that the development of the notochord is impaired is evident also at later stages. Whereas the normal notochord vanishes from the vertebral bodies and is supposed to contribute to the formation of the nucleus pulposus, the notochord in the strong Pax-1 mutants persists as a rod-like structure up to the new-born stage. This can be seen in skeletal preparations of the Uns/un genotype most clearly (Fig. 5D). Uns homozygotes also display fused dorsal root ganglia (DRG) at day 18.5 p.c. (Fig. 3E). To some degree DRG fusions can be seen in all un alleles (data not shown). In early stages, up to around day 12.5 p.c., no fusions are detected. These arise later during fetal development, possibly as a consequence of the severe shortening of the trunk (Fig. 3B). all or most cells that participate in the formation of ventral vertebral structures have expressed Pax-1 in their early developmental stages. In Grüneberg’s original description of un, he stated that the intervertebral discs are larger than in normal mice (Grüneberg, 1954). This observation could not be confirmed in the present study. On the contrary, the annulus fibrosus is much reduced and cannot be observed in whole-mount skeletal preparations. The reason for this discrepancy is likely due to the fact that the anlagen of the intervertebral discs are less well defined and occupy a larger space in craniocaudal dimension in mutant embryos. These anlagen will not differentiate into proper discs, however. Our morphological observations and conclusions made from these, that are greatly facilitated by the allelic series DISCUSSION Pax-1 is required for normal development of ventral vertebral structures The proper formation of ventral vertebral structures requires Pax-1 function. Vertebral bodies and intervertebral discs are virtually absent in Pax-1-negative mice while the neural arches are fairly normal. This conclusion is obvious for the lumbar region of Uns/Uns mutants where ventral structures are completely missing, while other regions as the upper thoracic region present a more complex phenotype. Here the vertebrae have a ventral structure that connects the neural arches. Due to its form and small size we suggest that these are ventral neural arches and the perichordal tube contribution to this tissue, if any, is very small. Thus Pax-1-negative mice lack vertebral bodies at all levels of the vertebral column down to the beginning of the tail. The tail phenotype of Uns/Uns mice is different, however, due to the fact that the ventral cartilage remnants are not continuous with the paired lateral ones. The different phenotype in the tail may represent a different mechanism for vertebra formation by cells originating from the tail bud. When the perichordal tube has acquired a welldefined segmentation at about day 12.5 p.c., the intervertebral disc anlagen are Pax-1-positive whereas the vertebral body anlagen are negative. It may not be surprising, however, that both vertebral bodies and intervertebral discs are heavily affected in the mutants since the immunostaining data indicate that Fig. 8. Notochord morphology and proliferation rate are changed in Pax-1deficient embryos. In lumbar transverse sections of day 13.5 +/+ (A) and Uns/Uns (B) embryos, the typical phenotype with absent ventral vertebral structures can be observed in the mutant. Moreover, the size of the notochord (arrowhead) is increased in the mutant. Higher magnification of +/+ (C) and Uns/Uns (D) reveals a significant increase in notochord diameter and cell number. Note also the difference in the notochordal sheaths (arrows), the mutant sheath being thinner. The larger size of the notochord can be attributed to a higher notochordal proliferation rate at day 12.5 p.c. When comparing transverse sections of un/+ (E) and Uns/un (F) embryos, a higher number of cells in the mutant notochord (n) have been labelled with BrdU. The figure shows two representative sections through the lumbar region. Scale bars: (A,B) 100 µm, (C,D) 10 µm, (E,F) 20 µm. Pax-1 and axial skeleton development 1119 now available, are otherwise in good agreement with Grüneberg’s work on un (1950, 1954). The role of Pax-1 in intervertebral disc development might represent a late function compared to the patterning of early sclerotome cells. In later stages, Pax-1 expression is found in disc anlagen and lining the skeletal structures. Pax-1 may here have a role in the differentiation of fibrous tissue, and therefore, in the maintenance of the boundaries between skeletal elements. Indeed, fusions between axial skeletal elements are frequently seen in the mutants. The strong phenotypic similarities in the three Pax-1 mutants leave little doubt that undulated is due to a reduction of Pax-1 function. While in un the phenotype can be linked to a single point mutation in the Pax-1 gene, the deletion sizes of the other two alleles are not known. It thus remains an open question whether other deleted genes also significantly contribute to the strong phenotype seen in Uns/Uns mice. We believe this not to be the case, because of the apparent lack of any phenotypic changes that cannot be observed in a milder form in un mice. This will not be strictly proven, however, until a clean Pax-1 null mutation has been accomplished via homologous recombination in ES cells and introduced into the germline or until Uns/Uns mice have been rescued via transgenesis. We suggest that Uns/Uns mice display a Pax-1 null phenotype and that the weaker mutations are hypomorphs. An observation in support of that idea is that Pax-1un protein still retains DNA-binding activity, albeit much weaker than the wild-type protein (Chalepakis et al., 1991). Further support for this notion is provided by the demonstration that the causative genetic change in the Splotch-delayed mutant, is a point mutation in the paired domain, similar to the situation in un.; the phenotype of this mutant was interpreted to be due to partial loss of Pax-3 function (Vogan et al., 1993). Recently, a detailed study of the interaction of paired domains with DNA target sequences revealed that this DNA binding domain is composed of two subdomains (Czerny et al., 1993). These authors discuss that a mutation in one of the subdomains may affect the binding to only a subset of target genes. This speculation is of great interest for the present study, as un is one of the potential models. Indeed, a differential effect on separate targets is consistent with our phenotypic analyses. Although all abnormalities to some degree can be seen in all mutants, the relative strength of phenotypic alterations in different regions are not always proportional. Thus, when the phenotype of un is compared to that of Uns, it can be seen that the point-mutation in un affects development in axial sclerotome derivatives relatively more than lateral ones, i.e. the rib/transverse process phenotype is fairly weak in un. We have recently identified a Pax gene that is highly homologous to Pax-1 (Wallin et al., 1993). This gene, Pax-9, is expressed in a similar but not identical pattern during sclerotome development. To analyse whether the expression of this gene is dependent on Pax-1 we have compared Pax-9 expression domains in wild-type and Uns/un embryos (A. Neubüser, J. W. and R. B., unpublished observations). We have not been able to detect any changes in expression patterns that may not primarily be due to morphological changes. Sclerotome and notochord are interdependent structures A common denominator of the affected vertebral structures is their proximity to the notochord. The development of the vertebral column is dependent on the presence of the notochord (Watterson et al., 1954), and a supernumerary notochord induces the formation of additional vertebral body-like structures, but represses myotome formation, in surgically manipulated chicken embryos (Brand-Saberi et al., 1993; Pourquie et al., 1993). Interestingly, it was also shown that the notochord is able to induce Pax-1 expression in dorsal somite derivatives, that are normally Pax-1 negative (Brand-Saberi et al., 1993). The induced Pax-1 expression is accompanied by a loss of dermomyotome cells and the appearance of sclerotome-like mesenchymal cells. These observations make Pax-1 a very good candidate for a mediator of notochordal signals to the sclerotome, and as such might function as an embryonic competence factor. Based on the analysis of Pax-1 expression in the notochord mouse mutant Danforth’s short-tail (Sd) and comparison of the Sd and un phenotypes, we have suggested that Pax-1 might be one of the major mediators of inductive signals from the notochord to the sclerotome (Koseki et al., 1993). Our observation that vertebral body formation is often differentially affected on either side of the midline is correlated with a displacement of the notochord to the more normal side. We suggest that the development of the bodies is dependent on notochord signals that come significantly later than the factor(s) that initially induce Pax-1 expression. The simplest interpretation would be that sustained notochord signalling is required for normal maintenance of Pax-1 expression, which in turn controls the downstream cascade involved in the formation of vertebral bodies and intervertebral discs. Alternatively, Pax-1 acts as a competence factor in sclerotome cells enabling them to respond to further notochord signals. A strong rib phenotype was observed in Uns/Uns. The proximal part is absent or reduced while lateral rib structures are unaffected. This division corresponds fairly well to those parts of the ribs that are apparently derived from medial and lateral somite-halves, respectively; the medial half gives rise to proximal ribs, whereas the lateral half gives rise to the more distal part of the rib (Charles Ordahl, personal communication). It may be that only the medial somite-half is dependent on notochord signals; in Sd, the rib phenotype is similar to the one in Uns/Uns (Koseki et al., 1993). Mutant mice with a phenotype that is virtually the opposite of that for Uns with respect to the ribs have been recently described (Braun et al., 1992). These mice carry targeted mutations in the myf-5 gene. To our surprise the morphological changes in the mutant also involve the notochord. Enlarged notochords were shown to have increased proliferation rates, and abnormal development results in the persistence of the notochord in vertebrae. These observations indicate that proper notochord development is dependent on surrounding sclerotome cells, which means that signalling may be bidirectional. Normally the notochord expands significantly at the level of the intervertebral discs, whereas it vanishes from the centres of the vertebral bodies. We suggest that notochordal cell proliferation is normally suppressed by surrounding perichordal tube cells and that subsequent differentiation of intervertebral discs leads to an expansion of the notochord only at these levels. A schematic model for the induction, expression and functional role of Pax1 is presented in Fig. 9. Another unexpected finding was the fusion of dorsal root ganglia. It is conceivable that this abnormality in a neural tissue 1120 J. Wallin and others Fig. 9. A schematic model for the induction of Pax-1 expression and the role of Pax-1 in vertebral column development. (A) Notochord signals induce Pax-1 expression in the ventromedial part of the somite (Brand-Saberi et al., 1993; Koseki et al., 1993). (B) Pax-1-expressing sclerotome cells give rise to ventral parts of the vertebral column. In this developmental process, there is a bidirectional dependence of cells of the notochord and the anlagen of the vertebral bodies and intervertebral discs for their proper differentiation (arrows). (C) The major consequences of a Pax-1 deficiency are absence of vertebral bodies and intervertebral discs (black) and the loss of proximal rib structures (grey) or rib homologues, i.e. the transverse processes. The neural arches (white) are relatively unaffected. is secondary to sclerotome abnormalities. It is well established that only the cranial half of each lateral sclerotome segment is permissive for neural crest cell migration and dorsal root ganglion growth (Keynes and Stern, 1984). In transplantation experiments in the avian system, it has been shown that neural crest-derived cell development in the context of cranial half somites only resulted in nonsegmented coalesced ganglia (Kalcheim and Teillet, 1989). In the Pax-1 mutants, the abnormal sclerotome cells in the cranial compartment may affect ganglion growth in a way akin to the influence on the notochord. Alternatively, the shortening of the vertebral column may cause a mechanical compression that forces the ganglia together, resulting in fusions. Pax genes and organ development From our observations in un mutant mice, we speculate that Pax-1 may act as an embryonic differentiation factor, making ventral mesenchymal cells competent to differentiate into hyaline and fibrous cartilage. Several mutant phenotypes resulting from genetic alterations in Pax genes have been described in diverse species (Chalepakis et al., 1992; Gruss and Walther, 1992; Noll, 1993), making the Pax gene family one of the currently better understood groups of developmental genes. Studies on the different Pax mouse mutants have documented a central role for Pax genes in morphogenesis. Splotch, a Pax-3 mutant, displays overgrowth of the neural tube with exencephaly and spina bifida, affecting also the migration of pigment cells, derivatives of the neural crest (Auerbach, 1954). Small-eye embryos, which carry a mutation in the Pax-6 gene, also display neural crest cell migration problems (Matsuo et al., 1993; Schmahl et al., 1993). Our data on the undulated phenotype might be interpreted as a problem of sclerotome cell proliferation, differentiation and/or migration. The significant reduction of ventral sclerotome cells has not been correlated to a readily detectable decrease in sclerotome cell proliferation rate (J. W. and R. B., unpublished observations). These preliminary data are, however, compatible with a slight but significant change in proliferation rate. Compared to the notochord, which displayed a large increase in proliferation rate, the developing sclerotome is a complex structure with regional differences in all three dimensions. An extended analysis is therefore required to document changes in the different sclerotome regions. One study that argues in favour of at least some proliferative role of Pax genes is the demonstration of an oncogenic activity of these genes (Maulbecker et al., 1993). Recently, a role for Pax-2 in the conversion of mesenchyme to epithelium during kidney development was demonstrated in an organ culture system (Rothenpieler and Dressler, 1993). This situation, where early mesenchymal cells fail to aggregate and differentiate, bears a strong resemblance to the phenotype in undulated mice where mesenchymal sclerotome cells fail to condense and initiate chondrogenesis. We propose that Pax-1 plays a crucial role in the early differentiation of ventral sclerotome cells, leading to profound changes in morphology and extracellular matrix composition. Very little is known about downstream target genes under control of Pax transcription factors. So far only three candidate target genes have been suggested; CD19 for Pax-5 and thyroperoxidase and thyroglobulin for Pax-8 (Kozmik et al., 1992; Zannini et al., 1992). The identification of Pax-1 as an essential gene for early sclerotome development provides a starting point, however, for a characterization of the cascade of events that underlie the morphogenesis of the axial skeleton. We thank Peter Gruss for the gift of Pax-1 cDNA, Mary Dickinson and Andy McMahon for the BrdU-labelling protocol, Bärbel Strack, Monika Schüttoff, Günther Frank and Sybille Antoni for technical assistance, Uli Birsner for oligonucleotide synthesis and Lore Lay for photographic work. J. W. was supported by an EMBO fellowship and the Swedish Medical Research Council. H. K. was supported by the Human Frontier Science Program. The work was supported by the Max-Planck-Society and the Deutsche Forschungsgemeinschaft (Ba 869/3-1). REFERENCES Adams, B., Dörfler, P., Aguzzi, A., Kozmik, Z., Urbanék, P., Maurer-Fogy, I. and Busslinger, M. (1992). Pax-5 encodes the transcription factor BSAP and is expressed in B lymphocytes, the developing CNS and adult testis. Genes Dev. 6, 1589-1607. Auerbach, R. (1954). Analysis of the developmental effects of a lethal mutation in the house mouse. J. Exp. Zool. 127, 305-329. Bagnall, K. M. (1992). The migration and distribution of somite cells after labelling with the carbocyanine dye, DiI: the relationship of this distribution to segmentation in the vertebrate body. Anat. Embryol. 185, 317-324. Balling, R., Deutsch, U. and Gruss, P. (1988). Undulated, a mutation affecting the development of the mouse skeleton, has a point mutation in the paired box of Pax 1. Cell 55, 531-535. Balling, R., Lau, C. F., Dietrich, S., Wallin, J. and Gruss, P. (1992). Development of the skeletal system. Ciba Foundation Symposium 165, 132145. Chichester: Wiley. Pax-1 and axial skeleton development 1121 Blandova, Y. R. and Egorov, I. U. (1975). Sut allelic with un. Mouse News Lett. 52, 43. Bopp, D., Burri, M., Baumgartner, S., Frigerio, G. and Noll, M. (1986). Conservation of a large protein domain in the segmentation gene paired and in functionally related genes of Drosophila. Cell 47, 1033-1040. Brand-Saberi, B., Ebensperger, C., Wilting, J., Balling, R. and Christ, B. (1993). The ventralizing effect of the notochord on somite differentiation in chick embryos. Anat. Embryol. 188, 239-245. Braun, T., Rudnicki, M. A., Arnold, H. H. and Jaenisch, R. (1992). Targeted inactivation of the muscle regulatory gene myf-5 results in abnormal rib development and perinatal death. Cell 71, 369-382. Chalepakis, G., Fritsch, R., Fickenscher, H., Deutsch, U., Goulding, M. and Gruss, P. (1991). The molecular basis of the undulated/Pax-1 mutation. Cell 66, 873-884. Chalepakis, G., Tremblay, P. and Gruss, P. (1992). Pax genes, mutants and molecular function. J. Cell Sci. 16 (suppl.), 61-67. Christ, B. and Wilting, J. (1992). From somites to vertebral column. Ann. Anat. 174, 23-32. Czerny, T., Schaffner, G. and Busslinger, M. (1993). DNA sequence recognition by Pax proteins: bipartite structure of the paired domain and its binding site. Genes Dev. 7, 2048-2061. Deutsch, U. (1990). Identifizierung der Paired-Box-Genfamile der Maus und Charakterisierung von Pax-1 als Entwicklungs-Kontrollgen. InauguralDissertation. Heidelberg. Deutsch, U., Dressler, G. R. and Gruss, P. (1988). Pax1, a member of a paired box homologous murine gene family, is expressed in segmented structures during development. Cell 53, 617-625. Epstein, D. J., Vekemans, M. and Gros, P. (1991). Splotch (Sp2h), a mutation affecting development of the mouse neural tube, shows a deletion within the paired homeodomain of Pax-3. Cell 67, 767-774. Ewan, K. B. R. and Everett, A. W. (1992). Evidence for resegmentation in the formation of the vertebral column using the novel approach of retroviralmediated gene transfer. Exp. Cell Res. 198, 315-320. Grüneberg, H. (1950). Genetical studies on the skeleton of the mouse. II. Undulated and its modifiers. J. Genet. 50, 142-173. Grüneberg, H. (1954). Genetical studies on the skeleton of the mouse. XII. The development of undulated. J. Genet. 52, 441-455. Grüneberg, H. (1963). The Pathology of Development. Oxford: Blackwell Scientific. Gruss, P. and Walther, C. (1992). Pax in development. Cell 69, 719-722. Hill, R. E., Favor, J., Hogan, B. L. M., Ton, C. C. T., Saunders, G. F., Hanson, I. M., Prosser, J., Jordan, T., Hastie, N. D. and van Heyningen, V. (1991). Mouse Small eye results from mutations in a paired-like homeobox-containing gene. Nature 354, 522-525. Kalcheim, C. and Teillet, M.A. (1989) Consequences of somite manipulation on the pattern of dorsal root ganglion development. Development 106, 8593. Kessel, M., Balling, R. and Gruss, P. (1990). Variations of cervical vertebrae after expression of a Hox-1.1 transgene in mice. Cell 61, 301-308. Kessel, M. and Gruss, P. (1991). Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell 67, 1-20. Keynes, R. J. and Stern, C. D. (1984). Segmentation in the vertebrate nervous system. Nature 310, 786-789. Koseki, H., Wallin, J., Wilting, J., Mizutani, Y., Kispert, A., Ebensperger, C., Herrmann, B. G., Christ, B. and Balling, R. (1993). A role for Pax-1 as a mediator of notochordal signals during the dorsoventral specification of vertebrae. Development 119, 649-660. Kozmik, Z., Wang, S., Dörfler, P., Adams, B. and Busslinger, M. (1992). The promoter of the CD19 gene is a target for the B-cell-specific transcription factor BSAP. Mol. Cell. Biol. 12, 2662-2672. Maulbecker, C. C. and Gruss, P. (1993). The oncogenic potential of Pax genes. EMBO J. 12, 2361-2367. Matsuo, T., Osumi-Yamashita, N., Noji, S., Ohuchi, H., Koyama, E., Myokai, F., Matsuo, N., Taniguchi, S., Doi, H., Iseki, S., Ninomiya, Y., Fujiwara, M., Watanabe, T. and Eto, K. (1993). A mutation in the Pax-6 gene in rat small eye is associated with impaired migration of midbrain cells. Nature Genet. 3, 299-304. Noll, M. (1993). Evolution and role of Pax genes. Curr. Opin. Genet. Dev. 3, 595-605. Pourquie, O., Coltey, M., Teillet, M.-A., Ordahl, C. and Le Douarin, N. (1993). Control of dorsoventral patterning of somitic derivatives by notochord and floor plate. Proc. Natl. Acad. Sci. USA 90, 5242-5246. Rosen, B. and Beddington, R. S. P. (1993). Whole-mount in situ hybridization in the mouse embryo: gene expression in three dimensions. Trends Genet. 9, 162-167. Rothenpieler, U. W. and Dressler, G. R. (1993). Pax-2 is required for mesenchyme-to-epithelium conversion during kidney development. Development 119, 711-720. Sambrook, J., Fritsch, E. F. and Maniatis, T. (1989). Molecular Cloning. A Laboratory Manual. Cold Spring Harbour NY: Cold Spring Harbour Laboratory Press Schmahl, W., Knoediseder, M., Favor, J. and Davidson, D. (1993). Defects of neuronal migration and the pathogenesis of cortical malformations are associated with Small eye (Sey) in the mouse, a point mutation at the Pax-6 locus. Acta Neuropathol. 86, 126-135. Theiler, K. (1988). Vertebral malformations Adv. Anat. Embryol. Cell Biol. 112, 1-99. Verbout, A. J. (1985). The development of the vertebral column. Adv. Anat. Embryol. Cell Biol. 90, 1-122. Vogan, K. J., Epstein, D. J., Trasler, D. G. and Gros, P. (1993). The Splotchdelayed (Spd) mouse mutant carries a point mutation within the paired box of the Pax-3 gene. Genomics 17, 364-369. Wallace, M. E. (1985). An inherited agent of mutation with chromosome damage in wild mice. J. Heredity 76, 271-278. Wallin, J., Mizutani, Y., Imai, K., Miyashita, N., Moriwaki, K., Taniguchi, M., Koseki, H. and Balling, R. (1993). A new Pax gene, Pax-9, maps to mouse chromosome 12. Mammalian Genome 4, 354-358. Walther, C., Guenet, J.-L., Simon, D., Deutsch, U., Jostes, B., Goulding, M., Plachov, D., Balling, R. and Gruss, P. (1991). Pax: A murine multigene family of paired box containing genes. Genomics 11, 424-434. Watterson, R. L., Fowler, I. and Fowler, B. J. (1954). The role of the neural tube and notochord in development of the axial skeleton of the chick. Am. J. Anat. 95, 337-397. Wright, M. E. (1947). Undulated: A new genetic factor in Mus musculus affecting the spine and tail. Heredity 1, 137-141. Zannini, M., Francis-Lang, H., Plachov, D. and Di Lauro, R. (1992). Pax-8, a paired domain-containing protein, binds to a sequence overlapping the recognition site of a homeodomain and activates transcription from two thyroid-specific promoters. Mol. Cell. Biol. 12, 4230-4241. (Accepted 8 February 1994)