* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Physical Chemistry 20130517 week 7 Friday May 17 2013
Survey
Document related concepts
Symmetry in quantum mechanics wikipedia , lookup
Elementary particle wikipedia , lookup
Tight binding wikipedia , lookup
Renormalization wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Electron scattering wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Hydrogen atom wikipedia , lookup
Wave–particle duality wikipedia , lookup
Matter wave wikipedia , lookup
Particle in a box wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Atomic theory wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Transcript
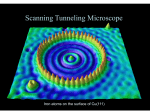
Physical Chemistry 20130517 week 7 Friday May 17, 2013 page 1 tunneling: scanning tunneling microscope (STM), important for analyzing surfaces, electrons tunnel from tip to surface electron tunneling proton tunneling hydrogen atoms tunneling α particle tunneling Tunneling is a quantum phenomenon. It has no equivalent in classical physics. A piezoelectric substance converts pressure to voltage or voltage to pressure. Voltage makes it expand and contract. 2 particle rigid rotor V = potential energy = 0 One mass is m1, the other mass is m2, the distance from the center of m1 to the center of m2 is r. The book calls r d instead. These particles can’t vibrate. dV=-Fdr=0 since the radius doesn’t change energy = KE + rotational energy Erot =J(J+1) ℏ2 2I (no potential energy) I is moment of inertia J=0,1,2,3… μ=reduced mass= I=μr 2 for diatomic molecule m1 m2 m1 +m2 energy levels J=3 J=2 J=1 J=0 E=12 E=6 E=2 E=0 The E in that table is really Notice the spacing between energy levels isn’t constant. polar coordinates E to simplify. ℏ2 ( 2I ) Consider a graph with mass m1 at the origin and mass m2 somewhere else. The distance between the two masses is r. The angle between the z axis and the radius r is θ. Project r down onto the xy plane gives a vector with length rsinθ. The angle between the x axis and the vector rsinθ is φ. Given that, we know: x=rsinθcosφ yrsinθsinφ z=rcosθ ψrot = ΘθΦ(φ) where Θ and Φ are functions The formula doesn’t include r since r doesn’t change. Θ depends on J and MJ. Φ depends on MJ. MJ is the component of J along the z axis. J = 0,1,2,3,… For J = 2 MJ can be -2, -1, 0, 1, 2. For a given J there are 2J+1 values for MJ. For J=1: MJ=+1 ψrot=ψ11 MJ=0 Ψrot=ψ10 MJ=-1 ψrot=ψ1-1 Therefore each energy level J has 2N+1 degeneracy. See approximation methods handout. For n=1: E= E(trial) = ∫φ*Ĥφdτ ≥ Egs n2 h2 h2 h2 = =.125 8ma2 8ma2 ma2 Egs is ground state energy Ĥ is the time independent Hamiltonian Both φ and φ* are already normalized. If they weren’t normalized, use this: ∫ φ*Ĥφdτ ≥Egs ∫ φ*φdτ Suppose we approximate ψ(x) using x(x-a). We want to show that it fits the boundary conditions for a particle in a box: lim x(x-a) =0 x→a and ̂ ψ=x(x-a) ψH lim x(x-a)=0 x→0 -h2 d2 x(x-a) 8π2 m dx 2 In this case the complex conjugate and the function are the same since they’re real. a ̂ ψdx= ∫ ψH 0 a a a -h2 -h2 -h2 a3 a3 2 ∫ x(x-a)dx = x dx -a ∫ xdx = (∫ ) ( - ) 4π2 m 0 4π2 m 0 4π2 m 3 2 0 = -h2 -a3 h 2 a3 = 4π2 m 6 24π2 m That’s not normalized, so take the integral of the bottom too. a a a a a ∫ ψ2 dx = ∫ x 2 (x-a)2 dx = ∫ x 4 dx +a2 ∫ x 2 dx -2a ∫ x 3 dx = 0 0 0 0 0 a5 a5 a 5 a5 + - = 5 3 2 30 h 2 a3 ( ) 30h2 a3 5h2 .127h2 24π2 m E' = a = = = = ma2 a5 24π2 ma5 4π2 ma2 ∫0 ψψdx ( ) 30 a ∫0 ψĤψdx % error= .127-.125 *100%=1.6% error (high by quantum mechanics standards) .125 Next we’ll try approximating ψ with x2(x-a)2