* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download BM Stem cell
Survey
Document related concepts
Transcript
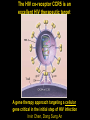
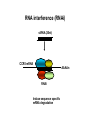
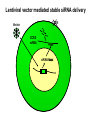
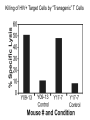
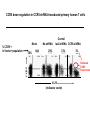
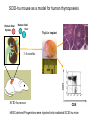
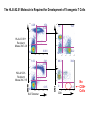
Engineering Stem Cells to Combat HIV Disease Jerome A. Zack Ph.D. David Geffen School of Medicine At UCLA What We Will Talk About HIV life cycle how the virus infects targets of current drugs Why gene therapy? What hematopoietic (blood forming) stem cells are A gene therapy strategy tested in the clinic Several strategies under development Human embryonic and induced pluripotent stem cells Viral RNA gp120 p24 RT & other virion proteins Fusion & Entry Binding CCR5 CD4 CXCR4 Reverse transcription Nuclear localization & entry Integration Viral RNA gp120 p24 Cellular Activation Assembly RT & other virion prteins Post-translational processing Budding Translation Viral Gene Transcription Why Gene Therapy? Current therapies are not 100% effective Resistance is seen, even to combined approaches Toxicities may preclude use of certain antiretrovirals Genetic therapies would target different aspects of The viral life cycle These types of therapies may be long-lasting, requiring only A single, or limited number of treatments Toxicities may be minimal Stem Cell Gene Therapy ES/iPS Cells Anti-Viral Gene Myeloid stem cell Erythroid progenitor Megakaryoblast Eosinophil progenitor Lymphoid stem cell BM Stem cell Basophil progenitor Myelomonocytic progenitor B progenitor T progenitor DP Thymocyte Monocyte NK Cell Megakaryocyte HIV CD8+ T cell Red blood cells Platelets CD4+ T cell Eosinophil Basophil Neutrophil Macrophage B cell HIV HIV Phase II Ribozyme Adult Stem Cell Gene Transfer Protocol An anti-viral gene therapy approach Hammerhead Ribozyme Cleavage site 5' 3' GGAGCCAGUA GAUCCUAA TARGET RNA RIBOZYME CUCGGUCA CUAGGAUU5' A CU G A A G A U Complementary G Complementary C G Flanking Sequence Flanking Sequence A U G C Catalytic Domain G C • RNA, hybridising arms A G • True enzymes - catalytic G U 3' domain • Nucleophilic attack after GUA Haseloff & Gerlach, 1988 Protocol Design Main Entry Criteria • 1st or 2nd ART regimen • Viral load < 400 c/ml for 6 Mo • CD4+ cells > 300/mcL • Age 18 - 45 years G-CSF Precursor cells • Randomised - active vs. placebo • Double blind • 2 yr study • 37 patients per group Infuse cells HIV-Infected individual CD34+ cells 2 x ART Interruptions (ATI) • 25 - 28 weeks • 41 - 48 weeks Rz 1o endpoint at 48 wk • Difference in Viral RNA at 48 wks Gene Therapy for HIV Disease Published online, Feb 15, 2009 74 adult HIV+ patients enrolled Largest cell-delivered gene therapy trial ever done Bone marrow stem cells from half the patients were treated with an anti-viral gene Half of the patients received control untreated stem cells Results Some diminishment in viral rebound when taken off of anti-retrovirals in treated group CD4+ T cell counts somewhat higher in treated group No adverse events due to gene therapy The HIV co-receptor CCR5 is an excellent HIV therapeutic target A gene therapy approach targeting a cellular gene critical in the initial step of HIV infection Irvin Chen, Dong Sung An Natural HIV resistance by CCR532/32 mutation CCR5 32/32 homozygous mutation 1% in Caucasian population No CCR5 expression Naturally protected from HIV-1 infection CCR5 32 heterozygous mutation 10% in Caucasian population 50% less CCR5 expression Slower progression to AIDS (2-3 years) These individuals have apparently normal health status Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation Hutter et.al. N Engl J Med. 2009 Feb 12;360(7):692-8. CCR5-32/ 32 BM Donor (HIV-) HLA matched Acute Myeloid Leukemia Patient (HIV+) BM transplant Following conditioning TBI Nearly 100% replacement with the CCR5 negative donor cells. HAART was discontinued after BM transplant. HIV RNA and DNA became undetectable at 68 days post-transplant and remained negative for 20 months. (now 3 years) RNA interference (RNAi) siRNA (20nt) CCR5 mRNA AAAAn RNAi Induce sequence specific mRNA degradation Lentiviral vector mediated stable siRNA delivery CCR5 Vector CCR5 mRNA siRNA This approach has thus far shown: long-term engraftment in monkeys Efficacy in mouse/human chimeric models An analogous approach is being pursued at City of Hope/USC This involves a reagent known as a zinc finger nuclease The concept is to add the nuclease to stem cells ex vivo, and this deletes the CCR5 gene. The stem cells will then be re-introduced into the patient Genetic Engineering of Human Immune Responses A stem cell genetic approach to enhance anti-viral immunity Scott Kitchen, PhD Stem Cell Gene Therapy Class I Restricted TCR Gene Myeloid stem cell Erythroid progenitor Megakaryoblast Eosinophil progenitor Lymphoid stem cell BM Stem cell Basophil progenitor Myelomonocytic progenitor B progenitor T progenitor DP Thymocyte Monocyte NK Cell Megakaryocyte CD8+ T cell Red blood cells Platelets Eosinophil Basophil Neutrophil Macrophage B cell CD4+ T cell HIV Gag SL9-Specific T Cell Receptor Restricted to HLA-A2.01 The Chimeric Model System 2. Transduce with Anti-HIV TCR (SL9 Peptide Specific) Fetal Liver Irradiate CD34+ CD34+ CD34+ CD34+ CD34+ ESCESC ESCESC ESC SCID-hu HLA-A2.1+ 3-12 weeks Human Thymus 3. Analyze TCR Expression 1. Sort CD34+ CD8 Scott Kitchen Killing of HIV+ Target Cells by “Transgenic” T Cells The data show us: Stem cells can be engineered to become anti-viral T cells These cells kill virally infected cells The TCR must “match” the donor HLA molecules This provides proof-of-principle that we can engineer the human immune system. Due to the mutation rate of the virus, for this approach to be valid for HIV disease, multiple TCRs specific for multiple antigens, in the context of different HLA molecules would be needed. We are currently testing this type of approach for human melanoma Which should not mutate as quickly as HIV, and be a better target A Word About Totipotent Stem Cells The previous studies all involved hematopoietic stem cells (HSC) These are applicable for stem cell therapeutics However, it may be difficult to obtain them, some patients will have poor quality stem cells, and these cells are difficult to expand Totipotent cells have some potential advantages over HSC Human embryonic stem cells (hESC) can be expanded in vitro These cells can be genetically manipulated easily There are no issues with difficulty of extraction from patients We have shown that they can be differentiated into T cells Induced Pluripotent Stem Cells (iPS) These cells have similar properties/advantages to hESC However they can be obtained from any patient, and will thus be genetically matched to the recipient, and not be rejected by the immune system. These cells also have the potential to differentiate along hematopoietic lineages. Conclusions Stem cell based therapies have been tested in the clinic, and have relevance to HIV disease Stem cell based therapeutics could offer life-long benefit, as stem cells themselves survive for the life of the individual These approaches are continually evolving Approaches attacking viral gene products, cellular gene products and that manipulate the immune system are in development It is likely that ablation of existing stem cell components (I.e. bone marrow) will be needed to increase the efficiency of reconstitution of newly introduced cells Development of ES and iPS technology may facilitate genetic therapeutic approaches to a variety of diseases CCR5 down-regulation in CCR5 shRNA transduced primary human T cells Control Mock % CCR5 + in Vector +population 30% CCR5 30.3 No-shRNA N/A 0.019 lacZ-shRNA CCR5-shRNA 29% 16.4 17.6 33% 30.8 4.31 3% 29.1 1.38 Reduced CCR5 Expression 69.7 0.019 23 43.1 56 EGFP (indicates vector) 8.9 30.1 39.4 Reduction of virus production in CCR5 tropic HIV-1 infected CCR5-shRNA transduced T cells in vitro p24 (ng/ml) 200 150 100 50 0 mock No-shRNA lacZshRNA CCR5shRNA CCR5 tropic HIV inhibition in human splenocytes ex vivo CCR5 tropic HIV CXCR4 tropic HIV 18 shRNA no shRNA 50 the amount of p24 (ng/mL) the amount of p24 (ng/ml) 60 40 30 20 10 shRNA no shRNA 16 14 12 10 8 6 4 2 0 0 0 2 4 6 8 days 10 12 14 0 2 4 6 8 days 10 12 14 T Cell Selection Lineage Commitment MHC I MHC II CD8+ CD4+ SCID-hu mouse as a model for human thymopoiesis Human fetal thymus Human fetal liver Thy/Liv implant 3-4 months SCID-hu mouse CD8 hESC-derived Progenitors were injected into irradiated SCID-hu mice CD8+ TCR expressing cells are made and exported to the periphery Thymus 10 3 50.5 0.25 10 2 Untransferred Control Mouse #17 6.7 10 3 79.5 10 1 10 1 10 0 10 0 0.12 10 0 10 1 10 2 10 3 13.1 2 10 1 10 0 11.7 10.6 1.89 73.3 10 0 10 1 SL9 Tetramer 10 2 0.003 10 3 2 10 2 10 1 10 1 10 0 10 0 3 10 0.48 10 1 10 2 93.8 10 CD8 0.06 0.01 5.6 3 10 0 10 3 SL9 Tetramer 10 10 0.003 99.95 CD8 3 10 4.03 9.79 SL9 Tetramer 10 0.04 10 2 0.2 49.1 HIV-TCR Transduced CD34+ Recipient Mouse #24 Spleen 0 10 1 10 2 10 0.01 99.72 3 0.21 10 0 10 1 SL9 Tetramer 10 2 10 3 The HLA*A2.01 Molecule is Required for Development of Transgenic T Cells HLA-A*2.01+ Recipient Mouse # 47-29 3 10 3 10 2 10 2 10 1 10 1 10 0 10 10 0 80.2 10 3 HLA-A*2.01Recipient Mouse # 47-15 15.4 15.4 4.28 0.061 10 0 10 1 10 2 10 3 2 10 2 10 1 10 1 0 10 0 10 10 10 SL9 Tetramer 1 10 2 10 0 10 1 10 69.3 2 3 10 3 30.6 0 0.15 0 42.2 10 10 95.4 15.8 12 10 3 3.41 3.35 1.02 30 0.1 10 CD8 0 10 1 10 2 10 3 No CD8+ Cells Analysis of HIV-Specific TCR on Transgenic T Cells SCID-hu Biopsy Thymocytes T1 cells (A2.1+) T1 cells (A*0201+) “load” with SL-9 peptides T1 cells (A2.1+) T1 cells (A2.1+) Mix, 1 Week Culture w/ IL-2 IFN- ELISPOT (3 additional days), Killing of targets