* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download CXCL10 Inhibits Viral Replication Through Recruitment of Natural
Survey
Document related concepts
Immune system wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Rheumatic fever wikipedia , lookup
Adaptive immune system wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Major urinary proteins wikipedia , lookup
DNA vaccination wikipedia , lookup
Infection control wikipedia , lookup
Molecular mimicry wikipedia , lookup
Neonatal infection wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Innate immune system wikipedia , lookup
Transcript
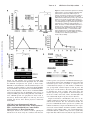
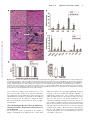
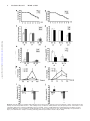
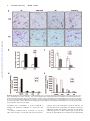
CXCL10 Inhibits Viral Replication Through Recruitment of Natural Killer Cells in Coxsackievirus B3-Induced Myocarditis Ji Yuan, Zhen Liu, Travis Lim, Huifang Zhang, Jianqing He, Elizabeth Walker, Courtney Shier, Yinjing Wang, Yue Su, Alhousseynou Sall, Bruce McManus, Decheng Yang Downloaded from http://circres.ahajournals.org/ by guest on August 9, 2017 Abstract—Coxsackievirus (CV)B3 is the primary cause of viral myocarditis. We previously observed CXC chemokine ligand 10 (CXCL10) upregulation in the myocardium early in infection. However, the impact of CXCL10 in CVB3-induced myocarditis is unknown. Using isolated primary mouse cardiomyocytes we demonstrated for the first time that cardiomyocytes can express CXCL10 on interferon-␥ stimulation. To explore the role of CXCL10 in CVB3-induced myocarditis, both CXCL10 transgenic and knockout mice were used. Following CVB3 challenges, the viral titer in the hearts inversely correlated with the levels of CXCL10 at early phase of infection before visible immune infiltration. Furthermore, as compared with the control mice, the decreased virus titers in the CXCL10 transgenic mouse hearts led to less cardiac damage and better cardiac function and vice verse in the knockout mice. This antiviral ability of CXCL10 might be through recruitment of natural killer (NK) cells to the heart and increased interferon-␥ expression early in infection. At day 7 postinfection, with massive influx of mononuclear cells the expression of CXCL10 enhanced the infiltration of CXCR3⫹ cells, CD4⫹, and CD8⫹ T cells, as well as the expression of associated inflammatory cytokines. However, the augmented accumulation of these immune cells and associated cytokines failed to alter the viral clearance and mice survival. These results suggest the protective role of CXCL10 during the early course of CVB3 infection, which is attributed to the recruitment of NK cells. Nonetheless, CXCL10-directed chemoattractant effect is not sufficient for host to clear the virus in the heart. (Circ Res. 2009;0:00-00.) Key Words: 䡲 cardiac function 䡲 cardiomyocytes 䡲 cardiomyopathy 䡲 chemokine 䡲 coxsackievirus 䡲 monocytes 䡲 myocarditis 䡲 natural killer cells C to mediate leukocyte migration to the site of infection.7 CXC chemokines, including interferon (IFN)-inducible protein 10 (IP10/CXCL10), monokine induced by IFN-␥ (Mig/CXCL9), and interferon-inducible T-cell a chemoattractant (I-TAC/ CXCL11), exert chemotactic effects on various mononuclear cells expressing the CXCR3 receptor.8 Among these chemokines, CXCL10 has been widely studied and is known to be involved in the regulation of lymphocyte recruitment observed in autoimmune inflammatory lesions,9 delayed-type hypersensitivity,10 many viral infections,11 and certain tumors. In a number of viral disease models, CXCL10 and its receptor CXCR3 have been shown to function in host resistance to virus infection by regulating the trafficking of activated inflammatory T cells,10 whereas other studies have reported that CXCL10 has no effect on T-cell migration and viral clearance.12,13 The important role of CXCL10 in the innate immune response has also been found to inhibit viral replication at early stage of infection through modulating natural killer (NK) cells trafficking and the delivery of NK cell-derived IFN-␥.14 In addition, expression of CXCL10 oxsackievirus (CV)B3 has been recognized as the predominant cause of viral myocarditis in humans.1 This disease is composed of three distinct stages including viremic injury, immune infiltration, and reclamation.2 Earlier studies have suggested that mechanisms of viral myocarditis include direct myocyte injury by CVB3 and subsequent immune-mediated damage of the heart.3,4 The essential role of the immune response in combating viral myocarditis has been demonstrated by recent studies using a series of knockout (KO) mice. Conversely, others have argued that the robust protective response can also be deleterious to host tissue to some extent. For instance, mice lacking T cells or T cell subsets developed less severe disease following CVB3 inoculation.5 However, general immunosuppressive therapy did not benefit patients with myocarditis,6 raising the need for better understanding the role of major immune mediators in disease cascade of CVB-induced myocarditis. During the process of leukocyte trafficking, it is well known that chemokines are the principle chemotactic factors Original received December 10, 2008; resubmission received December 10, 2008; accepted January 13, 2009. From the Department of Pathology and Laboratory Medicine, the iCAPTURE Centre, Heart an Lung Institute, University of British Columbia, Vancouver, Canada. Correspondence to Dr Decheng Yang, the iCAPTURE Center, University of British Columbia, St. Paul’s Hospital, 1081 Burrard St, Vancouver, BC, Canada V6Z 1Y6. E-mail [email protected] © 2009 American Heart Association, Inc. Circulation Research is available at http://circres.ahajournals.org DOI: 10.1161/CIRCRESAHA.108.192179 1 2 Circulation Research Month ●, 2009 has been found to contribute to the direct antimicrobial effect.14,15 These diverse reports on the role of CXCL10 may be attributable to the differences in the virus-host systems used. Whether CXCL10 plays a beneficial or detrimental role to the host in CVB3-induced myocarditis has not been studied. We previously found that CXCL10 was significantly upregulated in CVB3-infected murine heart by differential mRNA display and cDNA microarray.16,17 To better understand the role of CXCL10 in host immune responses against CVB3 infection, we conducted comparative studies using both a myocardium-specific CXCL10 transgenic (Tg) and a CXCL10 KO mouse model. Materials and Methods Generation of Transgenic Mice Downloaded from http://circres.ahajournals.org/ by guest on August 9, 2017 The murine CXCL10 gene was inserted between the murine ␣-myosin heavy chain (␣-MHC) promoter and human growth hormone poly(A) tail in the plasmid pBSII-SK (Figure 3a). The transgene was excised with Not 1 before microinjection of the pronuclei of 1-cell mouse embryos, which were reimplanted into pseudopregnant mice. The Tg mice were identified by PCR, ELISA and Western blot (see the online data supplement, available at http://circres.ahajournals.org). The KO mice were provided by Dr Andrew Luster (Harvard Medical School). Murine Cardiomyocyte Isolation and Culture Murine ventricular myocytes were isolated from 5- to 6-week-old male A/J mice and the detailed method has been described previously.18 The cardiomyocyte HL-1 cell line was a gift from Dr William C. Claycomb (Louisiana State University). Histological Analysis Five-week-old male mice (CXCL10 Tg and wild-type [Wt] littermates, CXCL10 KO and Wt) were infected intraperitoneally with 105 pfu of virus or PBS, and mice were euthanized at day 3, 7, and 10 postinfection (pi). Midventricular tissues were sectioned and stained with hematoxylin/eosin (H&E). Real-Time Quantitative RT-PCR Total mouse RNA was isolated from the heart using RNeasy kit (Qiagen). Real-time quantitative (q)RT-PCR was performed on an ABI Prism 7900HT Sequence Detection System using the premade primers and probes (Applied Biosystems) according to the instructions of the manufacturer. Enzyme-Linked Immunosorbent Assay Mouse serum was collected for determination of mouse cardiac troponin (cTn)I using a High Sensitivity Mouse Cardiac Troponin-I ELISA kit from Life Diagnostics. The assay was conducted according to the instructions of the manufacturer. Immnohistochemistry Paraffin-embedded heart sections were probed for the presence and localization of CD3 and CD45 using an ant-CD3 (DAKO) or an anti-CD45 antibody (BD Bioscience). The signals were detected using the substrates diaminobenzidine (for CD45 detection) (Sigma) and vector red (for CD3 detection) (Vector Laboratories). In Situ Hybridization Paraffin-embedded tissue sections were permeabilized with proteinase K, dehydrated, and hybridized with a digoxigenin-labeled CVB3 probe prepared by in vitro transcription as previously described.19 Flow Cytometry Hearts were digested with collagenase type II and trypsin (Sigma). Single-cell suspensions were prepared following the method pub- lished previously.16 Antibodies used for flow cytometry were: PE-CXCR3 from R&D and APC-CD45, APC-CD49b (NK cell), PE-CD3, PE-CD4, and PE-CD8 from eBioscience. Cells were run on a flow cytometer (Epics XL; Beckman Coulter Inc) and data were analyzed using Summit software (version 3.1; DakoCytomation). Echocardiography Mice were anesthetized with 2% Isoflurane and echocardiograms were recorded using a Vevo 770 (VisualSonics) system following the procedures described elsewhere.19 Measurements were taken for left ventricular (LV) end volumes at both diastole (LVEDV) and systole (LVESD). Cardiac Output (CO), and LV ejection fraction (LVEF) representing stroke volume as percentage of LVEDV were calculated by the Vevo 770 internal software. Results Upregulation of CXCR3 Chemokines After CVB3 Infection We found that IFN-␥ was induced as early as day 1 pi and elevated significantly between day 5 to 7 pi, indicating that it is expressed by both myocardial cells and infiltrating immune cells following viral infection. To determine the effect of CVB3 infection on the expression of CXCR3 chemokines including CXCL10, CXCL9, and CXCL11, mouse heart RNAs were extracted at day 3 and 7 pi and chemokine mRNA levels were measured by real-time qRT-PCR. As shown in Figure 1a, all 3 chemokines significantly increased at day 3 and declined at day 7 pi, with the CXCL10 showing the highest level among all three CXCR3 chemokines. The peak expression of their receptor CXCR3 expression was delayed to day 7 pi. Next we assessed the production of CXCL10 mRNA and protein in the serum over the time course of myocarditis. CVB3 challenge resulted in a maximal level of CXCL10 protein at day 3 pi and continued the production up to day 14 pi (Figure 1b and 1c). Given the maximal inflammation of myocarditis and peak expression of CXCR3 at day 7 pi, the early increase of CXCL10 before the infiltration suggests that mainly resident cells of the heart are the sources of CXCL10, which serves to amplify inflammation and protection by attracting immune cells expressing CXCR3. IFN-␥ but Not CVB3 Induces CXCL10 Expression From Mouse Cardiomyocytes To determine whether cardiomyocytes can produce CXCL10, primary mouse ventricular cardiomyocytes were stimulated by IFN-␥ or CVB3 infection. As early as 4 hours post induction, CXCL10 mRNA from the cells dramatically increased as compared with mock-treatment (Figure 1d). Interestingly, CXCL10 cannot be induced by direct CVB3 infection (Figure 1d), suggesting myocytes in the heart produce CXCL10 on IFN-␥ stimulation to attract more immune cells to the site of infection. Similar results were obtained using the mouse cardiomyocyte HL-1 cell line (Figure 1e). Cardiac-Specific Overexpression of CXCL10 Gene in Tg Mice To investigate the effect of CXCL10 on the development of CVB3-induced myocarditis, a Tg mouse model specifically overexpressing CXCL10 in the heart was generated by microinjection of a transgene containing a ␣-MHC promoter, which enables the target of the transgene specifically to the heart Yuan et al CXCL10 in Viral Myocarditis 3 Figure 1. CXCR3 chemokine expression following CVB3 infection of mice. Real-time qRT-PCR was performed to measure the mRNA copies of CXCR3 chemokines and their receptor CXCR3 in the hearts (a), as well as the levels of CXCL10 mRNA at the indicated time points pi (b). CXCL10 protein in the serum was detected by ELISA (c). The copy numbers of mRNAs were normalized by a housekeeping gene hypoxanthine phosphoribosyltransferase 1 (HPRT1). Treated with IFN-␥ or CVB3, the levels of CXCL10 mRNA expression in primary mouse cardiomyocytes were evaluated by real-time and regular RT-PCR (d). HL-1 cells were treated with IFN-␥ or infected with CVB3. CXCL10 expression was detected by RT-PCR and Western blot (e). -Actin mRNA was used as a loading control. Data are presented as means⫾SE (*P⬍0.05). Downloaded from http://circres.ahajournals.org/ by guest on August 9, 2017 (Figure 2a). The offspring were genotyped by PCR using genomic DNA isolated from tail clips (Figure 2b). To confirm the CXCL10 overexpression was specific to mouse hearts, RT-PCR, in situ hybridization, and Western-blot analyses were performed. As shown in Figure 2c and 2d, Tg CXCL10 mRNA was predominantly expressed in the heart of CXCL10 Tg mice, but not in Wt littermates. The low level of CXCL10 mRNA expression in the lungs may be attributable to the presence of endogenous MHC gene in the wall of pulmonary veins. Furthermore, CXCL10 protein was upregulated in mouse heart as detected by Western blot (Figure 2e). The Tg mice bred normally and did not show obvious physical or behavioral abnormalities. CXCL10 Causes Spontaneous Leukocyte Infiltration and Alteration of the Transcription of IFN-␥ and Interleukin-10 but Cannot Induce Myocyte Injury or Heart Dysfunction Histological sections were stained to look for pathological alterations in the heart of Tg mice without viral infection. Cardiac-specific overexpression of CXCL10 resulted in spontaneous minor mononuclear cell infiltrates in perivascular and interstitial regions of the myocardium as compared to the control littermates (Figure 3a). The number of infiltrations was age-dependent, with the highest in older Tg mice, but barely any in 4-week-old mice. We used real-time qRT-PCR to determine the levels of CD45, CD3, CD4, CD8, and natural cytotoxicity receptor (NCR). As shown in Figure 3b, the levels of CD3, CD4, CD8, CD45, and NCR (NK cell) were substantially higher in Tg mice than in Wt mice. Despite mononuclear cell infiltration, there were no discernible pathological alterations in the heart of Tg mice without viral infection. We also determined whether the presence of CXCL10 altered the expression of cytokines and CXCR3 chemokines. Compared to Wt littermates, the expression levels of other CXCR3 chemokines (CXCL9, CXCL11) and their receptor CXCR3, IFN-␥, and counterinflammatory interleukin (IL)-10 cytokines in Tg heart were significantly higher than that in Wt mouse heart, but the expression levels 4 Circulation Research Month ●, 2009 Downloaded from http://circres.ahajournals.org/ by guest on August 9, 2017 Figure 2. Generation of CXCL10 Tg mice. a, Structure of transgene CXCL10 with an upstream murine ␣-MHC promoter and a downstream human growth hormone poly-A tail. b, PCR to detect integration of the CXCL10 gene into chromosomal DNA. PCR bands (438 bp) were obtained by using a pair of primers (arrows) described in Materials and Methods. c, RT-PCR to detect CXCL10 mRNA expression in different organs. d, In situ hybridization of CXCL10 mRNA in Tg and Wt mouse hearts. e, Western blot to detect CXCL10 protein expression in different organs of mouse. of the proinflammatory cytokines (TNF, IL-4, IL-5, IL-6, IL-12a) were similar (Figure 3c). Furthermore, the presence of leukocytes and upregulation of IFN-␥ and IL-10 in the myocardium did not result in myocyte injury or heart function impairment, as revealed by (1) cTnI levels, a serum marker of myocyte injury (Figure 4g and 4h); (2) echocardiography to measure heart ejection fraction (EF) (Figure 3d); and (3) heart mass/body weight (Figure 3e). These findings indicate that CXCL10 directs mainly T cells and NK cells to the myocardium, associated with minor defense immunity but it is insufficient to cause cardiomyocyte destruction. Constitutive Cardiac CXCL10 Expression or Absence of CXCL10 Does Not Affect the Severity of Myocarditis To determine whether overexpression or ablation of CXCL10 affects the severity of myocarditis in CVB3-infected mice, pathological scoring of H&E-stained heart tissues of Wt, Tg, and KO mice was conducted by an experienced cardiac pathologist. Taking both myocyte damage and immune inflammation into consideration, there appeared to be a similar pathological score of myocarditis for all four groups at day 7 pi. Furthermore, CXCL10 Tg, KO, and Wt mice did not show the difference in mortality at this time point (Figure 4a and 4b). CXCL10 Expression Inhibits CVB3 Replication, Protects Myocytes From Injury, and Attenuates Heart Function Deterioration From CVB3 Infection in the Early Phase To further understand the role of CXCL10 in viral replication, immune infiltration, and viral clearance, we next analyzed the cell injury and immune influx separately. To assess the contribution of CXCL10 to antiviral defense, viral titers in the hearts were examined. To overcome the genetic effect of mouse on the virus clearance, we used mice with the same genetic background in each corresponding group as a control. At day 3 pi, viral titer in the mouse hearts inversely correlates with the levels of CXCL10 (Figure 4c and 4d): viral titer was higher in CXCL10 KO and lower in CXCL10 Tg as compared with the Wt controls. However, at days 7 and 10 pi, viral clearance was not significantly different in infected CXCL10 Tg/KO mice compared to controls (Figure 4c and 4d). The plaque assay results were confirmed by real-time qRT-PCR to detect the levels of CVB3 RNA in the hearts (Figure 4e and 4f). Because the cTnI levels in the serum are a sensitive indicator of myocardial injury,20 we used cTnI as a marker to detect myocyte damage during the active proliferative phase after CVB3 infection. We found that the serum cTnI levels were changed in a time-dependent manner in CVB3-induced murine myocarditis. The cTnI was increased at day 3, peaked at day 7 and normalized at day 10 pi (Figure 4g and 4h). At days 3 and 7 pi, the levels observed in CXCL10 KO mice were significantly higher than those in Wt mice, whereas the levels in CXCL10 Tg were lower than those in Wt mice, indicating CXCL10 expression attenuates cell damage from virus infection. Because cTnIs start to rise within 3 to 4 hours after onset of myocardial necrosis and remain raised for 4 to 10 days because of a gradual degeneration of myofibrils with release of the troponin complex,21 it is expected that the accumulated cTnI levels at day 7 pi reflect the myocardium damage at early time points. To examine whether the alteration of myocyte injury and inflammation by CXCL10 subsequently affects cardiac function following CVB3 infection, we conducted echocardiography in the acute phase of infection. At 5 days pi, the Wt Yuan et al CXCL10 in Viral Myocarditis 5 Downloaded from http://circres.ahajournals.org/ by guest on August 9, 2017 Figure 3. Mononuclear cells infiltration in the myocardium of CXCL10 Tg mice in the absence of CVB3 infection. a, H&E staining shows normal heart morphology of Wt mice and 4-week-old CXCL10 Tg mice, whereas mononuclear cell infiltrations are present in perivascular and interstitial regions of the myocardium of the 6-month-old CXCL10 Tg mice. Arrows indicate the magnification of the infiltrations. b and c, Real-time qRT-PCR to detect expression of CD45, CD3, CD4, CD8, NCR (NK cell marker), and proinflammatory cytokines in Tg hearts of 6-month-old mice. The values from the Wt were set to 1, and the relative mRNA units represent the fold increase over Wt mice. d, In vivo echocardiographic analysis of cardiac EF in age-matched Tg and Wt mice. e, Heart mass /body weight ratio in CXCL10 Tg and Wt mice. Data are presented as means⫾SE (*P⬍0.05). mice experienced a slightly change in EF and CO as compared to the baseline. However, the infected CXCL10 Tg mice exhibited an increased EF and CO, whereas the infected CXCL10 KO mice exhibited a significantly decreased EF and CO as compared to the Wt control mice (Figure 4i and 4j). The echocardiographic measurements are shown in supplemental Table I. CXCL10 Expression Recruits NK Cell Infiltration and Increases IFN-␥ Expression at Early Phase of Acute Infection To determine the effects of CXCL10 on the profiles of immune cell infiltration, the mRNA levels of CD4, CD8, NCR, and cytokines were measured by qRT-PCR. As shown in Figure 5, the levels of NK cells, CXCR3, and IFN-␥ in CXCL10 Tg and KO mice were correlated with that of CXCL10. Although the levels of CD4 and CD8 of Tg mice also rose, this increase was largely attributable to the constitutive CXCL10 overexpression because these elevations of CD4 and CD8 existed even before viral infection (Figure 3b). In addition, among the mechanisms by which NK cells control viral infection is the secretion of antiviral cytokines such as IFN-␥. Therefore, these results indicate that the early inhibition of viral replication by CXCL10 might be through the increased NK infiltration and concerted IFN-␥ expression. 6 Circulation Research Month ●, 2009 Downloaded from http://circres.ahajournals.org/ by guest on August 9, 2017 Figure 4. CXCL10 expression inhibits CVB3 replication and in turn protects cardiomyocytes from damage. a and b, Survival rate of KO and Tg mice following CVB3 infection. c and d, Plaque assay of infectious CVB3 particles using the apical portions of the hearts. e and f, Real-time qRT-PCR to measure CVB3 RNA in the myocardium. Copies of CVB3 RNA were normalized by that of HPRT1. g and h, ELISA to determine cTnI concentrations at indicated days pi. i and j, Cardiac function of mice. Echocardiography was performed on day 5 pi to determine the changes of LVEF (i) and CO (j). Data are presented as means⫾SE (*P⬍0.05). Yuan et al CXCL10 in Viral Myocarditis 7 Downloaded from http://circres.ahajournals.org/ by guest on August 9, 2017 Figure 5. Transcriptional expression of CD4, CD8, and NCR (a and b) and proinflammatory cytokines (b and d) in hearts at day 3 pi. RNA was prepared from hearts of Tg, KO, and Wt mice and subjected to real-time qRT-PCR. The values from the Wt were set to 1, and the relative mRNA units represent the fold induction over Wt mice. Data are presented as means⫾SE (*P⬍0.05). CXCL10 Expression Enhances Immune Response at Inflammatory Stage of Acute infection As the infiltration of leukocytes into the infected site is one of the most important pathological characteristics of viral myocarditis, we next examined whether CXCL10 can alter the migration of leukocytes and the associated immune responses in the acute phase of viral infection. As shown in Figure 6a through 6c, at day 7 pi, CXCL10 Tg mice accumulated more CD45⫹ and CD3⫹ cells in the myocardium and CXCL10 KO mice accumulated fewer than in Wt controls. Additional phenotypic analysis of the infiltrating leukocytes in the heart of CXCL10 Tg or KO mice revealed that these cells are predominantly CD4⫹ and CD8⫹ T cells (also see CBC data in supplemental Table II), and the number of these cells correlated with the levels of CXCL10 in the hearts (Figure 6 and 6e). These results were further solidified by real-time qRTPCR (Figure 7a and 7c). We next examined by qRT-PCR whether the altered leukocyte recruitment was associated with altered expression of a number of cytokines or chemokines. As shown in Figure 7b, the marked increase in leukocyte infiltration was accompanied by upregulation of gene expression of IFN-␥, IL-10, and IL-12a. In contrast to Wt mice, lower expression levels of these cytokines and additional lower CXCL11, TNF-␣, and IL-6 were seen in the CXCL10 KO mice (Figure 7d). Despite altered influx of leukocytes into the heart at 7 days pi, viral clearance did not significantly change in CXCL10 Tg or KO mice (Figure 4c and 4d). These data indicate that CXCL10 expression is correlated with the recruitment of leukocytes into the myocardium of mice with viral myocarditis, but CXCL10-induced chemotaxis of leukocytes is not sufficient for host immune responses to clear the viruses. Discussion Local secretion of cytokines and chemokines by cardiomyocytes and infiltrated inflammatory cells over the course of CVB3 infection is important in determining the pathogenesis of viral myocarditis. Several cytokines and chemokines such as TNF-␣, IL-1␣, and MCP-1 are known to be produced in cardiomyocytes after CVB3 infection.22,23 However, whether cardiomyocytes are capable to express CXCL10 is unknown. Here we showed for the first time that CXCL10 can be induced by IFN-␥ but not by CVB3 in primary adult mouse cardiomyocytes. These results suggest that early expression of IFN-␥ stimulates cardiomyocytes and/or other resident cells in the myocardium, such as resident immune cells, 8 Circulation Research Month ●, 2009 Downloaded from http://circres.ahajournals.org/ by guest on August 9, 2017 Figure 6. Identification and quantification of infiltrating leukocytes. a, Immunostaining of CD45 and CD3 in mouse heart at day 7 pi. b and c, Quantification of CD45⫹ and CD3⫹ cells. The percentage areas stained with respective antibodies were determined from the mean of 10 images per heart section using Image Pro software. d and e, Quantification of total infiltrating mononuclear cells. On day 7 pi, hearts were collected and infiltrating mononuclear cells were isolated by Histopaque. The indicated positive cells were calculated from the total number of the isolated cells by multiplying the percentage of each population obtained by flow cytometry analysis. Data are presented as means⫾SE (*P⬍0.05). endothelial cells, or fibroblasts, to produce CXCL10 to modulate the leukocytes infiltration to control the virus infection. As known, CXCL10 induces chemotaxis of activated CD4⫹ Th1 and CD8⫹ T cells, NK cells and monocyte/mac- rophages, but not the neutrophils. Consistent with this, our data and others from recent studies revealed the spontaneous infiltrations of NK cells and CD4⫹ and CD8⫹ T cells during CXCL10 expression.12,24 This is further supported by the fact that the T cells infiltrations in CVB3-infected CXCL10 KO Yuan et al CXCL10 in Viral Myocarditis 9 Downloaded from http://circres.ahajournals.org/ by guest on August 9, 2017 Figure 7. Transcriptional expression of CD4, CD8, and NK (a and c) and proinflammatory cytokines (b and d) in the hearts at day 7 pi. RNA was prepared from hearts of Tg, KO and Wt mice and subjected to real-time qRT-PCR. The values from the Wt were set to 1, and the relative mRNA units represent the fold induction over Wt mice. Data are presented as means⫾SE (*P⬍0.05). mice were decreased. However, with expression of CXCL10 in astrocytes, the immune infiltrates in the CNS were dominated by the neutrophils but not by the CD4⫹ and CD8⫹ T cells.24 This discrepancy between studies implies that additional tissue-specific factors may affect the composition of infiltrates to a specific organ. Previous studies of CXCL10 Tg mouse models reported that the leukocyte infiltration does not cause proinflammatory cytokine mRNAs upregulation.12,24 In our Tg mice, CXCL10 expression in cardiomyocytes induced a number of cytokine (IFN-␥, IL-10) and chemokine (CXCL9, CXCL11) upregulation, along with the T cell and NK cell infiltrations. However, their protein levels in the serum were not detectable by ELISA, suggesting the upregulation was limited. Furthermore, no cardiac damage, functional changes, or cardiomyopathy was observed in our CXCL10 Tg mice. Despite the presence of leukocyte infiltrations and increased IFN-␥ and IL-10 mRNAs, the CXCL10 cardiac-specific transgene is not sufficient to cause myocarditis, indicating additional mediators are required for induction of an immune-mediated disease. CXCL10 participates in both innate and adaptive immunity by contributing to cell migration and activation. The important role of CXCL10 in the innate immune response has also been shown in studies of vaccinia virus and mouse hepatitis virus. It controls viral replication by recruiting and activating NK cells.14,25 In our study, the early peak of CXCL10 before inflammatory cell infiltration implies its important role in innate immunity of CVB3-induced myocarditis. The results showed that at day 3 pi, CXCL10 overexpression prevented the viral replication, whereas CXCL10 KO impaired the ability to efficiently control virus replication. The mechanism by which CXCL10 inhibits CVB3 replication early may be via premature killing the host of viruses as it is known that CXCL10 can induce cell apoptosis.17 In addition, CXCL10 levels were associated with NK cell infiltration and IFN-␥ expression in the myocardium. Following CVB3 infection, NK cells infiltrate the heart first and are the major early-stage producers of IFN-␥.26 One mechanism by which NK cells defense against viral infection is through the secretion of antiviral cytokines such as IFN-␥.27 Indeed, NK cells and IFN-␥ have previously been shown to play important roles in 10 Circulation Research Month ●, 2009 Downloaded from http://circres.ahajournals.org/ by guest on August 9, 2017 limiting CVB3 replication in the heart.28,29 However, the factor mediating this function is unknown. Here, we found for the first time that CXCL10 is such a mediator. The early CXCL10 expression following CVB3 infection may inhibit viral replication in the heart through recruitment of NK cells and associated production of cytokines such as IFN-␥. The upregulation of IFN-␥ produces a positive feedback loop on CXCL10 expression. In addition to the effect on NK cell trafficking, CXCL10 could exert a direct antiviral effect. As reported recently, CXCL10 efficiently prevented Dengue virus from binding to the virus to the cell surface, thereby blocking its entry and replication.11 In this way, CXCL10 may limit the spread of infection and contribute to host defense early during infection. However, whether CXCL10 has a direct antiviral role in CVB3 infection needs further investigation. Recent reports have demonstrated a critical role in adaptive immunity for CXCL10 in directing effector T-cell migration toward the site of infection, which then facilitates viral clearance.10,30,31 During the inflammatory stage of acute CVB3 infection (day 7 pi), it was also expected that overexpression or deficiency of CXCL10 should augment or impair the ability of target cells to attract T cells expressing CXCR3 to the heart and in turn affect the clearance of viruses. However, neither overexpression nor deficiency of CXCL10 significantly altered the viral clearance or mouse survival rate despite altered T-cell recruitment and expression levels of associated cytokines. Furthermore, no difference in the severity of myocarditis was observed between CXCL10 Tg or KO mice versus the controls by the histological evaluation. These results imply that CXCL10 is not an essential inflammatory-stage mediator compared to other chemokines, such as macrophage inhibitory protein (MIP)-1␣. For example, mice KO MIP-1␣ are resistant to CVB3-induced myocarditis.32 Anti–MIP-2 antibody treatment decreased cellular infiltrations and myocardial necrosis and increased survival rate than the control group.33 Notably, both MIP-1␣ and MIP-2 peaked around day 7 to 10 pi when the maximal inflammatory changes occurred, unlike CXCL10. The distinctive expression patterns of these chemokines imply their different roles in the course of CVB3 infection. At day 7 pi, there was no difference in the severity of myocarditis by pathological scoring. Interestingly, further quantitative analyses of cell damage and immune infiltration showed that Tg mice had less accumulated cell damage but more immune infiltration, whereas KO mice had opposite results as compared to the control mice. This may lead to the similar pathological grades among groups when both parameters were considered. Furthermore, the early transient antiviral effect of CXCL10 in the hearts could not rescue the mice from death. This may be attributable to, firstly, the high dose of virus (1⫻105 pfu) in infection. However, this is a dose widely used in studies of pathogenesis of CVB3 and many other virus infections of mice. In another experiment, when we infected mice with 103 pfu, we observed differential effect of CXCL10 on survival rates of KO and Wt mice (Figure I in the online data supplement). Secondly, mice infected by CVB3 were possibly died of multiple organ dysfunctions.2 Here, as we focused on viral myocarditis we did not inves- tigate the role of CXCL10 in other organ diseases following virus infection. It is unknown whether CXCL10 affects the viral replication and inflammation in other susceptible organs, such as liver and pancreas. Collectively, following CVB3 infection, early rise of IFN-␥ stimulates CXCL10 expression in cardiomyocytes and other resident myocardial cells. CXCL10 expression inhibits CVB3 replication at early stage and in turn protects cardiomyocytes from damage and improves heart function. This antiviral activity of CXCL10 is acquired through regulation of NK cell infiltration and associated INF-␥ expression, suggesting a critical role for CXCL10 during the early course of CVB3 infection. However, the transient antiviral effect is insufficient for viral clearance and rescuing the mice from death during acute inflammation stages. The other chemokines or cytokines, such as MIP-1␣ and TNF-␣, may also play an important role in the clearance of viruses. In other words, host immune responses against external invasion need the orchestrated action of multiple antiviral mediators to effectively protect host itself. Thus, early intervention of CVB3 by CXCL10 combined with other chemokines and/or cytokines during inflammation may provide a new therapeutic strategy toward viral-induced myocarditis. Sources of Funding This work was supported by grants from the Canadian Institutes of Health Research and Heart and Stroke Foundation of British Columbia and Yukon. Disclosures None. References 1. Maze SS, Adolph RJ. Myocarditis: unresolved issues in diagnosis and treatment. Clin Cardiol. 1990;13:69 –79. 2. McManus BM, Yanagawa B, Rezai N, Luo H, Taylor L, Zhang M, Yuan J, Buckley J, Triche T, Schreiner G, Yang D. Genetic determinants of coxsackievirus B3 pathogenesis. Ann N Y Acad Sci. 2002;975:169 –179. 3. Chow LH, Beisel KW, McManus BM. Enteroviral infection of mice with severe combined immunodeficiency. Evidence for direct viral pathogenesis of myocardial injury. Lab Invest. 1992;66:24 –31. 4. Henke A, Huber S, Stelzner A, Whitton JL. The role of CD8⫹ T lymphocytes in coxsackievirus B3-induced myocarditis. J Virol. 1995; 69:6720 – 6728. 5. Opavsky MA, Penninger J, Aitken K, Wen WH, Dawood F, Mak T, Liu P. Susceptibility to myocarditis is dependent on the response of alphabeta T lymphocytes to coxsackieviral infection. Circ Res. 1999;85:551–558. 6. Mason JW, O’Connell JB, Herskowitz A, Rose NR, McManus BM, Billingham ME, Moon TE. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med. 1995;333:269 –275. 7. Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593– 620. 8. Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. 9. Fife BT, Kennedy KJ, Paniagua MC, Lukacs NW, Kunkel SL, Luster AD, Karpus WJ. CXCL10 (IFN-gamma-inducible protein-10) control of encephalitogenic CD4⫹ T cell accumulation in the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2001;166:7617–7624. 10. Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–3204. Yuan et al Downloaded from http://circres.ahajournals.org/ by guest on August 9, 2017 11. Chen JP, Lu HL, Lai SL, Campanella GS, Sung JM, Lu MY, Wu-Hsieh BA, Lin YL, Lane TE, Luster AD, Liao F. Dengue virus induces expression of CXC chemokine ligand 10/IFN-gamma-inducible protein 10, which competitively inhibits viral binding to cell surface heparan sulfate. J Immunol. 2006;177:3185–3192. 12. Rhode A, Pauza ME, Barral AM, Rodrigo E, Oldstone MB, von Herrath MG, Christen U. Islet-specific expression of CXCL10 causes spontaneous islet infiltration and accelerates diabetes development. J Immunol. 2005;175: 3516–3524. 13. Tsunoda I, Lane TE, Blackett J, Fujinami RS. Distinct roles for IP-10/ CXCL10 in three animal models, Theiler’s virus infection, EAE, and MHV infection, for multiple sclerosis: implication of differing roles for IP-10. Mult Scler. 2004;10:26 –34. 14. Mahalingam S, Farber JM, Karupiah G. The interferon-inducible chemokines MuMig and Crg-2 exhibit antiviral activity In vivo. J Virol. 1999;73:1479 –1491. 15. Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, Strieter RM. Cutting edge: IFN-inducible ELR-CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001;167:623– 627. 16. Taylor LA, Carthy CM, Yang D, Saad K, Wong D, Schreiner G, Stanton LW, McManus BM. Host gene regulation during coxsackievirus B3 infection in mice: assessment by microarrays. Circ Res. 2000;87: 328 –334. 17. Zhang HM, Yuan J, Cheung P, Chau D, Wong BW, McManus BM, Yang D. Gamma interferon-inducible protein 10 induces HeLa cell apoptosis through a p53-dependent pathway initiated by suppression of human papillomavirus type 18 E6 and E7 expression. Mol Cell Biol. 2005;25: 6247– 6258. 18. Boyd JH, Mathur S, Wang Y, Bateman RM, Walley KR. Toll-like receptor stimulation in cardiomyoctes decreases contractility and initiates an NF-kappaB dependent inflammatory response. Cardiovasc Res. 2006; 72:384 –393. 19. Cheung C, Marchant D, Walker EK, Luo Z, Zhang J, Yanagawa B, Rahmani M, Cox J, Overall C, Senior RM, Luo H, McManus BM. Ablation of matrix metalloproteinase-9 increases severity of viral myocarditis in mice. Circulation. 2008;117:1574 –1582. 20. Smith SC, Ladenson JH, Mason JW, Jaffe AS. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation. 1997;95:163–168. 21. Bertinchant JP, Larue C, Pernel I, Ledermann B, Fabbro-Peray P, Beck L, Calzolari C, Trinquier S, Nigond J, Pau B. Release kinetics of serum cardiac troponin I in ischemic myocardial injury. Clin Biochem. 1996; 29:587–594. CXCL10 in Viral Myocarditis 11 22. Okuno M, Nakagawa M, Shimada M, Saito M, Hishinuma S, YamauchiTakihara K. Expressional patterns of cytokines in a murine model of acute myocarditis: early expression of cardiotrophin-1. Lab Invest. 2000;80: 433– 440. 23. Shen Y, Xu W, Chu YW, Wang Y, Liu QS, Xiong SD. Coxsackievirus group B type 3 infection upregulates expression of monocyte chemoattractant protein 1 in cardiac myocytes, which leads to enhanced migration of mononuclear cells in viral myocarditis. J Virol. 2004;78:12548 –12556. 24. Boztug K, Carson MJ, Pham-Mitchell N, Asensio VC, DeMartino J, Campbell IL. Leukocyte infiltration, but not neurodegeneration, in the CNS of transgenic mice with astrocyte production of the CXC chemokine ligand 10. J Immunol. 2002;169:1505–1515. 25. Trifilo MJ, Montalto-Morrison C, Stiles LN, Hurst KR, Hardison JL, Manning JE, Masters PS, Lane TE. CXC chemokine ligand 10 controls viral infection in the central nervous system: evidence for a role in innate immune response through recruitment and activation of natural killer cells. J Virol. 2004;78:585–594. 26. Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol. 2001;19: 65–91. 27. Huhn MH, Hultcrantz M, Lind K, Ljunggren HG, Malmberg KJ, Flodstrom-Tullberg M. IFN-gamma production dominates the early human natural killer cell response to Coxsackievirus infection. Cell Microbiol. 2008;10:426 – 436. 28. Godeny EK, Gauntt CJ. Murine natural killer cells limit coxsackievirus B3 replication. J Immunol. 1987;139:913–918. 29. Fairweather D, Yusung S, Frisancho S, Barrett M, Gatewood S, Steele R, Rose NR. IL-12 receptor beta 1 and Toll-like receptor 4 increase IL-1 beta- and IL-18-associated myocarditis and coxsackievirus replication. J Immunol. 2003;170:4731– 4737. 30. Hsieh MF, Lai SL, Chen JP, Sung JM, Lin YL, Wu-Hsieh BA, Gerard C, Luster A, Liao F. Both CXCR3 and CXCL10/IFN-inducible protein 10 are required for resistance to primary infection by dengue virus. J Immunol. 2006;177:1855–1863. 31. Klein RS, Lin E, Zhang B, Luster AD, Tollett J, Samuel MA, Engle M, Diamond MS. Neuronal CXCL10 directs CD8⫹ T-cell recruitment and control of West Nile virus encephalitis. J Virol. 2005;79:11457–11466. 32. Cook DN, Beck MA, Coffman TM, Kirby SL, Sheridan JF, Pragnell IB, Smithies O. Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science. 1995;269:1583–1585. 33. Kishimoto C, Kawamata H, Sakai S, Shinohara H, Ochiai H. Role of MIP-2 in coxsackievirus B3 myocarditis. J Mol Cell Cardiol. 2000;32: 631– 638. Downloaded from http://circres.ahajournals.org/ by guest on August 9, 2017 CXCL10 Inhibits Viral Replication Through Recruitment of Natural Killer Cells in Coxsackievirus B3-Induced Myocarditis Ji Yuan, Zhen Liu, Travis Lim, Huifang Zhang, Jianqing He, Elizabeth Walker, Courtney Shier, Yinjing Wang, Yue Su, Alhousseynou Sall, Bruce McManus and Decheng Yang Circ Res. published online January 22, 2009; Circulation Research is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 2009 American Heart Association, Inc. All rights reserved. Print ISSN: 0009-7330. Online ISSN: 1524-4571 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circres.ahajournals.org/content/early/2009/01/22/CIRCRESAHA.108.192179.citation Data Supplement (unedited) at: http://circres.ahajournals.org/content/suppl/2009/01/22/CIRCRESAHA.108.192179.DC1 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation Research can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Circulation Research is online at: http://circres.ahajournals.org//subscriptions/ Yuan et al. SUPPLEMENT MATERIAL CXCL10 INHIBITS VIRUS REPLICATION THROUGH RECRUITMENT OF NATURAL KILLER CELLS IN COXSACKIEVIRUS B3-INDUCED MYOCARDITIS Ji Yuan, Zhen Liu,Travis Lim, Huifang Zhang, Jianqing He, Elizabeth Walker, Courtney Shier, Yinjing Wang, Yue Su, Alhousseynou Sall, Bruce McManus, Decheng Yang* Department of Pathology and Laboratory Medicine, The iCAPTURE Centre, Heart + Lung Institute, University of British Columbia, Vancouver, British Columbia, Canada Expanded Materials and Methods Generation of transgenic mice The murine CXCL10 gene was excised from the pCDNA3/CXCL10 plasmid with EcoR I and cloned into plasmid pBSII-SK at the same site. The 5.5 kb fragment in the generated new plasmid containing in order the murine α-myosin heavy chain (α-MHC) promoter, the coding sequence of murine CXCL10 and human growth hormone poly(A) tail (Fig. 3a) was excised with Not 1 before microinjection. This α-MHC promoter located upstream of CXCL10 enabled heart-specific expression. The sequence was confirmed by DNA sequencing at the Nucleic Acid Protein Service Unit, University of British Columbia (UBC). C57BL/6 and CBA F1 hybrid mice were used for generation of transgenic mice. The transgene construct was microinjected into the pronuclei of one-cell mouse embryos, which 1 Yuan et al. were reimplanted into pseudopregnant mice. After offspring were born, the tails were biopsied and serum was collected at the age of 3 weeks and 6 weeks respectively. The founders were identified by PCR with a 5’ primer (5’AAGTGGTGGTGTAGGAAAG3’) specific to the α-MHC promoter and 3’ primer (5’AAGCTTCTAGTTAGTCAGTC3’) specific to CXCL10 after isolating genomic DNA from tail snips. Furthermore, sandwich ELISA was employed for determining CXCL10 expression levels in PCR-positive mice. The transgenic founders with highest CXCL10 expression levels were mated back to A/J mice for > six generations to introduce pure genetic A/J background. To confirm the CXCL10 upregulation in mouse heart, RT-PCR, ELISA and Western-blot analyses were performed. For CXCL10 knockout mice (Balb/C), further breeding was performed using breeder pairs kindly provided by Dr. Andrew Luster (Massachusetts General Hospital). Wild type Balb/C mice were purchased from Jackson Laboratories. Echocardiography Mice were anesthetized with 2% Isoflurane and echocardiograms were recorded using a Vevo 770® (VisualSonics) system. Echocardiographic measurements were taken from 2D M-mode left ventricular (LV) short axis view at the papillary muscle level. Mice were laid prone on a temperature-controlled stage and temperature monitored throughout the procedure. Views were standardized serially and between mice by strict adherence to the anatomical guidelines and conventions established by the American Society of Echocardiography. Measurements were taken for LV end volumes at both diastole (LVEDV) and systole 2 Yuan et al. (LVESD). Cardiac Output (CO), and LV Ejection Fraction (EF) representing stroke volume as percentage of LVEDV were calculated by the Vevo 770® internal software. Statistical analysis The results are expressed as means ± SE. Statistical analyses were performed with Student's t test. The values of P < 0.05 were considered statistically significant. 3 Supplemental Table I. Echocardiographic measurements in CXCL10 Tg and KO mice at indicated time points post CVB3 infection (pi). Items WT Tg WT Baseline Tg D5 pi HR ( per min) 494.78±1.51 435.75±18.73 468.33±22.56 428.5±24.47 LVIDd (mm) 3.81±0.29 3.58±0.37 3.53±0.40 3.50±0.49 LVIDs (mm) 2.79±0.34 2.58±0.36 2.45±0.44 2.35±0.53 EF (%) 55.64±3.01 64.30±7.23 47.52±4.42 64.47±5.61* CO (ml/min) 15.59±1.96 14.55±2.23 12.37±1.80 13.88±1.86* D14 pi D28 pi HR (per min) 417.21±68.56 386.01±52.56 456.20±51.25 402.32±36.46 LVIDd (mm) 3.52±0.45 3.35±0.42 3.83±0.32 4.01±0.14 LVIDs (mm) 2.38±0.48 2.33±0.30 2.69±0.34 2.73±0.15 EF (%) 62.14±10.66 58.77±7.34 57.64±5.41 60.63±2.54 CO (ml/min) 14.46±5.56 12.54±6.30 17.64±3.03 17.60±3.34 Items Wt KO Wt KO HR (per min) 392.33±20.94 420.25±11.46 349.44±22.11 389±23.67 LVIDd (mm) 3.43±0.09 3.69±0.10 3.25±0.15 3.61±0.33 LVIDs (mm) 2.45±0.08 2.82±0.07 2.41±0.17 3.12±0.11* EF (%) 56.00±2.85 47.81±1.04 56.50±5.96 28.25±4.76* CO (ml/min) 10.79±1.04 11.65±0.92 7.71±1.32 6.50±1.23 Baseline D5 pi HR=Heart rate; LVIDd=Left ventricular internal dimension in diastole; LVIDs= left ventricular internal dimension in systole; EF=ejection fraction; CO=cardiac output. * P<0.05. 4 Supplemental Table II. Complete blood count in CXCL10 Tg and KO mice at day 10 post CVB3 infection. ITEMS UNIT 10 days pi WT-Balb/c KO-Balb/c WT-AJ Tg-AJ WBC 10e9/L 5.26 3.45 3.93 4.92 NEU 10e9/L 2.51 0.71 1.66 1.62 LYM 10e9/L 2.42 2.00 1.74 2.46 MONO 10e9/L 0.27 0.65 0.47 0.80 EOS 10e9/L 0.02 0.01 0.01 0.01 BASO 10e9/L 0.04 0.08 0.05 0.03 RBC 10e12/L 9.69 10.05 9.01 8.62 HGB mmol/L 15.29 9.70 9.05 8.72 HCT L/L 0.45 0.47 0.44 0.43 MCV fL 46.16 46.45 48.79 49.91 MCH fmoL 1.60 0.97 1.01 1.02 MCHC mmol/L 34.71 20.83 20.64 20.34 RDW %CV 18.19 22.08 19.34 19.40 WBC=White blood cell; NEU=Neutrophil; LYM=Lymphocyte; MONO=Monocyte; EOS=Eosinophil; BASO=Basophil; RBC=Red blood Cell; HGB=Hemoglobin; HCT=Hematocrit; MCV=Mean corpuscular volume; MCH=Mean corpuscular hemoglobin; MCHC=Mean corpuscular hemoglobin concentration; RDW=RBC. The values are average of two experiments. 5 a. Survival rate % 120 KO 100 WT 80 60 40 20 0 d pi 1 2 3 4 5 6 7 8 9 b. 20 Wt KO 10 d pi 0 -10 -20 -30 ∆Cardiac output (%) ∆Ejection fraction (%) c. 0 d pi -10 -20 -30 Wt WT KO KO -40 -50 -60 -70 d5 d7 d10 d5 d7 d10 Supplemental Figure I. a). Survival curves of CXCL10 KO vs. WT mice following CVB3 infection. b). Ejection fraction change of mice at days 5, 7 and 10 post infection (d pi). c). Cardiac output change of mice at days 5, 7 and 10 pi. 6