* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Free-radical polymerization
Marcus theory wikipedia , lookup
Stöber process wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Click chemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Thermal spraying wikipedia , lookup
Rate equation wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Chemical reaction wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Shape-memory polymer wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Thermomechanical analysis wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Transition state theory wikipedia , lookup
Self-healing material wikipedia , lookup
Thermal runaway wikipedia , lookup
Microelectromechanical systems wikipedia , lookup
Hydroformylation wikipedia , lookup
Process chemistry wikipedia , lookup
Polyfluorene wikipedia , lookup
Sol–gel process wikipedia , lookup
Plasma polymerization wikipedia , lookup
Polythiophene wikipedia , lookup
Emulsion polymerization wikipedia , lookup
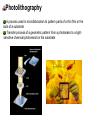
Photolytic Polymerization Review of radical, ionic polymerization Free-radical polymerization (chain reaction) - Initiators (direct method, Azo compounds, peroxides, or redox reactions) Ionic polymerization - Initiators (alkali metal suspensions and alkyl or aryllithium reagents) Photo polymerization can also be induced by supplying the initiation energy through irradiation with visible or ultraviolet light, high-energy or ionizing radiation, or by the passage of an electric current. 1. Photochemical polymerization 1.1 Advantages and Uses Avoidance of chemical contamination by initiator residues Convenience of photochemical reactions A wide range of reaction temperatures Utilization of photo polymerization and photo cross-linking for practical photographic process 1.2 Photopolymerization Process Light Monometer or un-cross-linked polymer Negative Base Solvent to remove unpolymerized or uncross-linked material Cross-linked polymer Relief image Figure_ Photopolymerization as photographic process. Illumination of a monomer or a cross-linkable polymer forms an insoluble relief image. 1.3 Monomers that undergo photopolymerization Any monomer that will undergo chain reaction polymerization is susceptible to photopolymerization or photosensitized polymerization. The absorption steps and the termination reactions are generally not affected. The advantage of photopolymerization and photosensitized polymerization is that the initiation process may take place over a wide range of temperatures and with a greater specificity than is found in chemically initiated systems. Other monomers, such as styrene or methyl methacrylate, are susceptible to direct photopolymerization when exposed to 300 nm or shorter wavelength light. 1.4 Experimental Technique: A Photosensitized Polymerization of Styrene 1.5 Mechanism of Initiation by Direct Photolysis of the Monomer Two different types of direct photoinitiation can be recognized. First, light absorption yields an electronically excited monomer molecule which subsequently decomposes to give radical fragments. Rate of initiation The rate of initiation of the chains and the dependence of this rate on the monomer concentration and temperature is quite different in photochemical initiator from the situation found in thermal initiation. Rate of initiation for thermal initiation Rate of initiation for photoinitiation The rate of initiating radical formation in a photoinitiation process is almost independent of the temperature but is proportional to the monomer concentration. Second, initiation mechanism is exemplified by the photopolymerization of styrene or methyl methacrylate. Fluorescence Diradical 1.6 Photosensitized polymerizations Photosensitizers are substances that produce free radicals when they absorb ultraviolet or visible light. The same substances that are used for thermal initiation are often used for photosensitization. Azo compounds and peroxides are photosensitizers, and the photoinitiaion reaction is the same as is the thermal initiation process. For example, azoisopropane does not dissociate sufficiently rapidly below 180 oC to be a useful thermal initiator. However, it photodissociates even at low temperatures when irradiated with near-ultraviolet light: 1.7 Photosensitizers for photopolymerization Photolithography A process used in microfabrication to pattern parts of a thin film or the bulk of a substrate Transfer process of a geometric pattern from a photomaks to a lightsensitive chemical photoresist on the substrate What is lithography? 석판화 기술, 인쇄 기술 Litho (돌) + graphy (그림, 글자) Recently Semiconductor manufacturing: Microlithography = transfer the pattern of circuits from a photomask to a wafer (a thin slice of silicon or other semiconductor material on which chips are made Lithography Analogy Camera Exposure instruments “Taking pattern on silicon wafer” 사진film Mask Photo paper (인화지) Wafer 감광제 Photo-resist감광제 햇빛, 조명 Laser light source Photo-resist A photoresist is a light-sensitive material used in several industrial processes, such as photolithography. Phenolic resins Epoxy-based polymer Process of photolithography