* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Introductory Chemistry, 2nd Edition Nivaldo Tro
Nuclear fusion wikipedia , lookup
Asymmetric induction wikipedia , lookup
Chemical potential wikipedia , lookup
Thermomechanical analysis wikipedia , lookup
Acid–base reaction wikipedia , lookup
Chemical bond wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Water splitting wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Biochemistry wikipedia , lookup
Process chemistry wikipedia , lookup
History of molecular theory wikipedia , lookup
Atomic theory wikipedia , lookup
Electrolysis of water wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Metalloprotein wikipedia , lookup
Marcus theory wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Thermodynamics wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Rate equation wikipedia , lookup
Electrochemistry wikipedia , lookup
Click chemistry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
George S. Hammond wikipedia , lookup
Stoichiometry wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Chemistry for Biology
Chapter 3
Chemical
Equilibrium
Btec , Biology dept, Guangdong
institute of Education
2009, Bio-department
Thermodynamics: Directionality
of Chemical Reactions
Josian W. Gibbs 1839-1903.
Pioneered concepts of chemical
thermodynamics and free energy.
Ludwig Boltzmann 1844-1906.
Famous for his equation
statistically defining entropy.
So far, we have tried to answer the following questions:
(1) What are the energetics (heat) of a reaction?
Is it exothermic (H= -) or endothermic (H= +)?
(2) How fast (kinetics) and how (mechanism) does the
reaction go?
(3) To what extent does it go? (equilibrium)
And finally now ……..
(4) Does it go, i.e., is it spontaneous?
This is the subject of this chapter.
Spontaneous processes: defined descriptively as a process
that occurs by itself (and the reverse does not occur by itself)
hot
heat
cold
The opposite:
is spontaneous,
hot
cold
heat
is not spontaneous,
but, it is possible (how does a refrigerator work?).
Other spontaneous processes (ask yourself: does reverse ever
occur by itself?)
nail rusting
eggs breaking (Humpty Dumpty)
paper burning
water freezing at -10oC
ice melting at +10oC
gases mix
All of these spontaneous processes are also described as:
irreversible
Irreversibility ═ Spontaneity
There are reversible processes, but the systems must be at
equilibrium.
heat + ice → water
at +10oC
Spontaneous, irreversible
heat + ice ← water
at -10oC
Spontaneous, irreversible
heat + ice water
at 0oC
Reversible; equilibrium
Both ice and water coexist at 0oC
Either process, → or ← can occur at equilibrium
What makes a process spontaneous (irreversible)?
G = H - TS
Free
energy
Enthalpy
Entropy
A
G<0
B
Exothermic reactions tend to be spontaneous
(exception, dissolving ammonium salts),
and increasing entropy (randomness) tends to cause
processes to be spontaneous; but overall
Gibbs Free Energy must decrease in order
for a process to be spontaneous.
examples:
Ice melting – similar to gas expansion – more randomness and
disorder, even though process is endothermic
Ink drops in water – ink becomes evenly distributed in water;
increase in randomness and disorder.
Decay of biological organisms – increase in randomness and
disorder.
Dissolving of salts in water – increase in randomness and disorder
To Summarize: what contributes to spontaneity?
1. Exothermic processes (heat is evolved).
2. Any process which increases randomness and disorder.
The thermodynamic quantity which describes randomness and
disorder is called ENTROPY and denoted as
S
The SECOND LAW OF THERMODYNAMICS postulates the
existence of entropy; it also states that the entropy of the universe
is constantly increasing. It is not a conserved quantity.
1. Gases have more entropy than liquids, which have more
entropy than solids.
2. Corollary: Melting, or vaporization, increases entropy.
3. Corollary: In a chemical rx., increasing the number of moles
of a gas, increases the entropy (e.g.,
H2O(g) H2(g) + ½O2(g)).
4. Dissolving or mixing increases entropy.
5. Corollary: precipitation decreases entropy.
6. Increasing the temperature increases entropy.
Thermodynamic Calculations
What is ΔG for the oxidation of SO2 to SO3 at 25°C? Is the
reaction spontaneous? exothermic?
SO2(g) + ½O2(g) SO3(g)
ΔHf° -296.8
0
-395.2
ΔS° 0.2485 ½(0.205)
0.2562
ΔH = -98.4 kJ
ΔS = -0.0948 kJ
ΔG = ΔH – TΔS = -98.4 – (298)(-0.0948)
= -98.4 + 28.2
= -70.15 kJ
The reaction is spontaneous.
The reaction is exothermic.
Note: Be sure you convert
ΔS values from J to kJ
What is ΔG for the decarboxylation of limestone at 25°C? Is the
reaction spontaneous? exothermic?
CaCO3(s) CaO(s) + CO2(g)
ΔHf° -1207.1
-635.5 -393.5
ΔH = +178.1 kJ
ΔS° 0.0929
0.0398 0.2136 ΔS = +0.1605 kJ
ΔG = ΔH – TΔS = +178.1 – (298)(+0.1605)
= + 178.1 - 47.83
= +130.27 The reaction is not spontaneous.
The reaction is endothermic.
Note: Be sure you convert
ΔS values from J to kJ
How do we make the decarboxylation of limestone spontaneous?
Set ΔG = 0, the the crossing over point where the reaction converts
from nonspontaneous to spontaneous.
ΔG = 0 = ΔH – TΔS
0 = +178.1 –T(+0.1605)
T = 1109K = 837℃
When the temperature falls below 837°C, CO2 begins
spontaneously to react with CaO to form CaCO3:
CaO(s) + CO2(g) CaCO3(s)
At room temperature, ΔG = + 130.27 kJ
Calculate the boiling point of methanol.
CH3OH(l) CH3OH(g)
ΔHf°
-238.7
-200.7
ΔS
+0.1268
+0.2398
ΔH = +38.0 kJ
ΔS = +0.113 kJ
At equilibrium, ΔG is always 0.
ΔG = 0 = ΔH – TΔS
=+38.0 –T(+0.113)
Tb = 336K = 63.3℃
Note: Be sure you convert
ΔS values from J to kJ
Additional aspects of Free Energy
• Even though a reaction has a negative G it may occur too
slowly to be observed (i.e. combustion).
• Thermodynamics gives us the direction of a spontaneous
process, it does not give us the rate of the process.
• A nonspontaneous process can be driven if coupled with a
spontaneous process – this is very important in life processes
(i.e., respiration to form ATP), and can be used in industrial
processes, such as smelting.
• To calculate K values, use ΔG° = -RT ln Keq.
This refers to the ΔG difference of the standard states of
compounds, before equilibrium is attained.
Laws of Thermodynamics
1st Law. Energy is neither created nor destroyed. In
chemistry, chemical energy can be converted into heat and
vice versa.
2nd Law. Entropy increases spontaneous; i.e., the natural
tendency is for randomization.
3rd Law. The entropy of a perfect crystal at 0K is zero (it is
impossible to attain 0K).
Reaction Rates
• Some chemical reactions proceed rapidly.
Like the precipitation reactions where the products
form practically the instant the two solutions are mixed.
• Other reactions proceed slowly.
Like the decomposition of dye molecules of a sofa
placed in front of a window.
• The rate of a reaction is measured in the amount of
reactant that changes into product in a given
period of time.
Generally moles of reactant used per second.
Like miles per hour.
• Chemists study ways of controlling reaction rates.
15
Reaction Rates, Continued
Initially, only reactants are present
After 15 seconds, the left reaction is 60% complete,
but the right reaction is only 20% complete
After 30 seconds, the left reaction is complete,
whereas the right reaction is only 40% done.
After 45 seconds, the right reaction is
still not complete
16
Collision Theory
•
•
In order for a reaction to take place, the reacting
molecules must collide with each other.
Once molecules collide they may react together
or they may not, depending on two factors:
1. Whether the collision has enough energy to “start to
break the bonds holding reactant molecules
together."
2. Whether the reacting molecules collide in the proper
orientation for new bonds to form.
19
Effective Collisions
• Collisions in which these two conditions are
met (and therefore the reaction occurs) are
called effective collisions.
• The higher the frequency of effective
collisions, the faster the reaction rate.
• There is a minimum energy needed for a
collision to be effective. We call this the
activation energy(活化能).
The lower the activation energy, the faster the
reaction will be.
20
Effective Collisions:
Kinetic Energy Factor
For a collision to
lead to overcoming
the energy barrier,
the reacting
molecules must have
sufficient kinetic
energy so that when
they collide, it can
form the activated
complex(活化复合体).
21
Effective Collisions:
Orientation Effect(定向效应)
22
Reaction Energy Diagram
23
Factors Effecting Reaction Rate:
Reactant Concentration
• The higher the concentration of reactant
molecules, this increases the frequency of
reactant molecule collisions the faster the
reaction will generally go.
• Since reactants are consumed as the reaction
proceeds, the speed of a reaction generally
slows over time.
24
Effect of Concentration on Rate
Low concentrations of reactant
molecules lead to fewer effective
collisions, therefore a slower
reaction rate.
High concentrations of reactant
molecules lead to more effective
collisions, therefore a faster
reaction rate.
25
Factors Effecting Reaction Rate:
Temperature
• Increasing the temperature increases the energy so
that their collisions can overcome the activation
energy.
• And, increasing the temperature also increases the
frequency of collisions.
• Both these mean that increasing temperature
increases the reaction rate.
26
Effect of Temperature on Rate
Low temperatures lead to fewer
molecules with enough energy to
overcome the activation energy,
and less frequent reactant collisions,
therefore a slower reaction rate
High temperatures lead to more
molecules with enough energy to
overcome the activation energy,
and more frequent reactant
collisions, therefore, a faster
reaction rate.
Activation Energy
• The energy barrier that prevents any
collision between molecules from being an
effective collision is called the activation
energy.
• The larger the activation energy of a
reaction, the slower it will be.
(屏障)
At a given temperature.
28
Relative potential energy
Exothermic Reaction
Activation
energy,
large
Activation
energy,
small
Reactants
Hreaction
Products
Progress of reaction
29
Relative potential energy
Endothermic Reaction
Activation
energy
Products
Hreaction
Reactants
Progress of reaction
30
Effect of Catalysts on Rate
• A catalyst( ) is a substance that increases
the rate of a reaction, but is not consumed in
the reaction.
• Catalysts lower the activation energy of a
reaction.
• Catalysts work by providing an easier
pathway for the reaction.
催化剂
31
Catalyst Effect on Activation
Energy
32
Catalyst Effect on Activation
Energy
33
Enzymes
• Enzymes( ) are protein molecules produced by
living organisms that catalyze chemical
reactions.
• The enzyme molecules have an active site
to
which organic molecules bind.
• When the organic molecule is bound to the active
site, certain bonds are weakened
• This allows a particular chemical change to occur
with greater ease and speed.
酶
(催化)
(活化位点)
(削弱).
i.e., the activation energy is lowered.
34
Chemical Equilibrium
• When a reaction reaches equilibrium, the
amounts of reactants and products in the
system stay constant.
• The forward and reverse reactions still continue.
• Because they go at the same rate, the amounts
of materials do not change.
37
Equilibrium
Initially, we only have reactant molecules in the
mixture. The reaction can only proceed in the
forward direction, making products.
Eventually, the forward and reverse rates are equal.
At this time equilibrium is established.
As the reaction proceeds, the forward reaction
slows down as the reactants get used up. At the
same time, the reverse reaction speeds up as
product concentration increases.
Once equilibrium is established, the
concentrations of the reactants and products in
the final mixture do not change, (unless
conditions are changed).
Equilibrium Equal
• The rates of the forward and reverse reactions are
equal at equilibrium.
• But that does not mean the concentrations of
reactants and products are equal.
• Some reactions reach equilibrium only after
almost all the reactant molecules are consumed—
we say the position of equilibrium favors the
products.
• Other reactions reach equilibrium when only a
small percentage of the reactant molecules are
consumed—we say the position of equilibrium
favors the reactants.
42
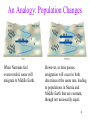
An Analogy: Population Changes
When Narnians feel
overcrowded, some will
emigrate to Middle Earth.
However, as time passes,
emigration will occur in both
directions at the same rate, leading
to populations in Narnia and
Middle Earth that are constant,
though not necessarily equal.
43
Equilibrium Constant
• Even though the concentrations of reactants
and products are not equal at equilibrium, there
is a relationship between them.
• For the reaction H2(g) + I2(g) 2HI(g) at
equilibrium, the ratio of the concentrations
raised to the power of their coefficients is
constant.
2
K eq
HI
H 2 I 2
44
Equilibrium Constant
• For the general equation aA + bB cC + dD,
the relationship is given below:
The lowercase letters represent the coefficients of
the balanced chemical equation.
Always products over reactants.
• The constant is called the equilibrium
constant, Keq.
c
d
C D
K eq
a
b
A B
45
Writing Equilibrium Constant
Expressions
• For aA + bB cC + dD,
the equilibrium constant
expression is:
C D
A a Bb
c
K eq
d
• So for the reaction
4
2 N2O5 4 NO2 + O2, the K NO2 O2
eq
N 2O5 2
equilibrium constant
expression is:
46
Equilibrium Constants for
Heterogeneous Equilibria
(异质的)
• Pure substances in the solid and liquid state have
constant concentrations.
Adding or removing some does not change the
concentration because they do not expand to fill the
container or spread throughout a solution.
• Therefore, these substances are not included in the
equilibrium constant expression.
For the reaction CaCO3(s) + 2 HCl(aq) CaCl2(aq) + CO2(g) + H2O(l):
K eq
CaCl 2 CO 2
HCl2
47
Write the Equilibrium Constant Expressions,
Keq, for Each of the Following:
• 2 CO2(g) 2 CO(g) + O2(g)
• BaSO4(s) Ba+2(aq) + SO4-2(aq)
• CH4(g) + 2 O2(g) CO2(g) + 2 H2O(l)
48
Write the Equilibrium Constant Expressions,
Keq, for Each of the Following, Continued:
• 2 CO2(g) 2 CO(g) + O2(g)
[CO]2•[O2]
Keq =
[CO2]2
• BaSO4(s) Ba+2(aq) + SO4-2(aq)
Keq =[Ba+2]•[SO4-2]
• CH4(g) + 2 O2(g) CO2(g) + 2 H2O(l)
[CO2] •[H2O]2
Keq = [CH ]•[O ]2
4
2
49
What Does the Value of Keq Imply?
• When the value of Keq > > 1, we know that when the
reaction reaches equilibrium, there will be many more
product molecules present than reactant molecules.
The position of equilibrium favors products.
• When the value of Keq < < 1, we know that when the
reaction reaches equilibrium, there will be many more
reactant molecules present than product molecules.
The position of equilibrium favors reactants.
50
A Large Equilibrium Constant
51
A Small Equilibrium Constant
52
Write the Equilibrium Constant Expressions,
Keq, and Predict the Position of Equilibrium
for Each of the Following:
• 2 HF(g) H2(g) + F2(g)
Keq = 1 × 10-95
• 2 SO2(g) + O2(g) 2 SO3(g)
Keq = 8 × 1025
• N2(g) + 2 O2(g) 2 NO2(g)
Keq = 3 × 10-17
53
Write the Equilibrium Constant Expressions, Keq,
and Predict the Position of Equilibrium for Each
of the Following:
• 2 HF(g) H2(g) + F2(g)
H 2 F2
K eq
2
HF
• 2 SO2(g) + O2(g) 2 SO3(g)
SO3 2
Keq
SO2 2 O2
• N2(g) + 2 O2(g) 2 NO2(g)
NO 2
K eq
2
N 2 O2
2
Keq = 1 × 10-95
Favors reactants.
Keq = 8 × 1025
Favors products.
Keq = 3 × 10-17
Favors reactants.
Calculating Keq
• The value of the equilibrium constant may be
determined by measuring the concentrations of all
the reactants and products in the mixture after the
reaction reaches equilibrium, then substituting in
the expression for Keq.
• Although you may have different amounts of
reactants and products in the equilibrium mixture,
the value of Keq will always be the same.
(取代)
The value of Keq depends only on the temperature.
The value of Keq does not depend on the amounts of
reactants or products with which you start.
55
Initial and Equilibrium Concentrations for
H2(g) + I2(g) 2HI(g)
Equilibrium
Constant
Equili
brium
Initial
[HI]
K
[H ][I ]
2
[H2]
[I2]
[HI] [H2]
[I2]
[HI]
eq
2
0.50
0.0
0.50
1.0
0.50
0.0
0.11
0.11
0.78
2
[0.78]2
50
[0.11][0.11]
0.50 0.055 0.055 0.39
[0.39]2
50
[0.055][0.055]
0.50 0.50 0.165 0.165 1.17
[1.17]2
50
[0.165][0.165]
0.0
0.5
0.0
0.53 0.033 0.934
[0.934]2
50
[0.53][0.033]
56
Example 3.3—Find the Value of Keq for the Reaction
from the Given Concentrations:
2 CH4(g) C2H2(g) + 3 H2(g).
Given: [CH4] = 0.0203 M, [C2H2] = 0.0451 M, [H2] = 0.112 M
Find: K
eq
Solution Map: [CH ], [C H ], [H ]
4
2 2
2
K eq
Relationships:
Solve:
Keq
Check:
Keq
[C2H 2 ] [H 2 ]3
[CH4 ]2
[C2H 2 ] [H 2 ]3 0.04510.112 3
0.154
2
[CH4 ]2
0.0203
Keq is unitless.
57
Practice—Calculate Keq for the Reaction
2 NO2(g) N2O4(g)
at 100 C if the Equilibrium Concentrations Are
[NO2] = 0.0172 M and [N2O4] = 0.0014 M.
58
Practice—Find the Value of Keq for the Reaction from the
Given Concentrations:
2 NO2(g) N2O4(g).
Given: [NO2] = 0.0172 M, [N2O4] = 0.0014 M
Find: K
eq
Solution Map:
[NO2], [N2O4]
K eq
Relationships:
Solve:
K eq
Check:
[N 2O4 ]
[NO 2 ]2
Keq
[N 2O 4 ] 0.0014
4.7
2
2
[NO 2 ]
0.0172
Keq is unitless.
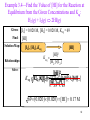
Example 3.4—Find the Value of [HI] for the Reaction at
Equilibrium from the Given Concentrations and Keq:
H2(g) + I2(g) 2HI(g)
Given: [I2] = 0.020 M, [H2] = 0.020 M, Keq = 69
Find:
Solution Map:
Relationships:
Solve:
[HI]
[I2], [H2], Keq
[HI]
K eq
[HI]2
[H 2 ] [I 2 ]
[HI]
[HI]22
2[H
Keq [H
[H
[I
]
[I] 2 ]
K eq2 ]K
]
[I
]
[HI]
2[]HI
2
eq22
[H22]] [I 22]]
[H
69 0.020 0.020 [HI ] 0.17 M
60
Disturbing and Re-Establishing
Equilibrium
• Once a reaction is at equilibrium, the
concentrations of all the reactants and products
remain the same.
• However, if the conditions are changed, the
concentrations of all the chemicals will change
until equilibrium is re-established.
• The new concentrations will be different, but the
equilibrium constant will be the same.
Unless you change the temperature.
61
Additional aspects of Free Energy
• Even though a reaction has a negative G it may occur too
slowly to be observed (i.e. combustion).
• Thermodynamics gives us the direction of a spontaneous
process, it does not give us the rate of the process.
• A nonspontaneous process can be driven if coupled with a
spontaneous process – this is very important in life processes
(i.e., respiration to form ATP), and can be used in industrial
processes, such as smelting.
• To calculate K values, use ΔG° = -RT ln Keq.
This refers to the ΔG difference of the standard states of
compounds, before equilibrium is attained.
Calculating Keq from ΔG values
Let’s calculate Keq from the following reaction, which we
previously studied in the Equilibrium chapter:
N2O4(g) 2NO2(g)
ΔGf°
98.3
2(51.8)
ΔG = 5.3 kJ
ΔG = -RT ln K
5300 = -8.314(407) ln K
ln K = -1.57
R = 8.314 J/mol-K
K = 0.208 -- very close to the
experimental value
Let’s take a look at the dissolution of NH4Cl.
NH4Cl(s) NH4+(aq) + Cl-(aq)
ΔHf°
ΔS°
-314.4
0.0946
-132.5
-167.2
ΔH = +14.7
0.1135
0.0565
ΔS = +0.0754
ΔG = ΔH – T ΔS = 14.7 – (298)(0.0754)
= 14.7 – 22.47
= -7.77 kJ
The reaction is endothermic, but is spontaneous!
Hence, ammonium chloride is excellent for cold packs.
Le Châtelier’s Principle
• Le Châtelier’s principle guides us in
predicting the effect on the position of
equilibrium when conditions change.
• “When a chemical system at equilibrium is
disturbed, the system shifts in a direction
that will minimize the disturbance.”
65
The Effect of Concentration
Changes on Equilibrium
• Adding a reactant will decrease the amounts of the other
reactants and increase the amount of the products until a
new position of equilibrium is found.
That has the same Keq.
• Removing a product will increase the amounts of the
other products and decrease the amounts of the reactants.
You can use to this to drive a reaction to completion!
• Remember: Adding more of a solid or liquid does not
change its concentration and, therefore, has no effect on
the equilibrium.
66
The Effect of Concentration Changes
on Equilibrium
When NO2
is added,
some of it
combines
to make
more N2O4.
67
The Effect of Concentration Changes
on Equilibrium
When N2O4
is added,
some of it
decomposes
to make
more NO2.
68
Practice—Predict the Effect on the Equilibrium
When the Underlined Substance Is Added to
the Following Systems:
• 2 CO2(g) 2 CO(g) + O2(g)
• BaSO4(s) Ba2+(aq) + SO42-(aq)
• CH4(g) + 2 O2(g) CO2(g) + 2 H2O(l)
69
Practice—Predict the Effect on the Equilibrium
When the Underlined Substance Is Added to
the Following Systems, Continued:
• 2 CO2(g) 2 CO(g) + O2(g)
Shift right, removing some of the added CO2 and
increasing the concentrations of CO and O2.
• BaSO4(s) Ba2+(aq) + SO42-(aq)
Shift left, removing some of the added Ba2+ and
reducing the concentration of SO42-.
• CH4(g) + 2 O2(g) CO2(g) + 2 H2O(l)
Shift right, removing some of the added CO2 and decreasing the O2,
70
while increasing the concentration of CO2.
Effect of Volume Change
on Equilibrium
• For solids, liquids, or solutions, changing
the size of the container has no effect on the
concentration.
• Changing the volume of a container
changes the concentration of a gas.
Same number of moles, but different number of
liters, resulting in a different molarity.
71
Effect of Volume Change
on Equilibrium
• Decreasing the size of the container increases the
concentration of all the gases in the container.
This increases their partial pressures.
• If their partial pressures increase, then the total
pressure in the container will increase.
• According to Le Châtelier’s principle, the
equilibrium should shift to remove that pressure.
• The way to reduce the pressure is to reduce the
number of molecules in the container.
• When the volume decreases, the equilibrium shifts
to the side with fewer molecules.
72
The Effect of Volume Change on Equilibrium
When
Sincethe
there
pressure
are more
is decreased
gas
molecules
by increasing
on the reactants
volume, the
side
position
of theof
reaction,
equilibrium
whenshifts
the
toward
pressure
the is
side
increased
with thethe
greater
position
number
of of
equilibrium
molecules—the
shifts
toward
reactant
the products.
side.
73
The Effect of Temperature
Changes on Equilibrium
• Exothermic reactions release energy and
endothermic reactions absorb energy.
• If we write heat as a product in an
exothermic reaction or as a reactant in an
endothermic reaction, it will help us use Le
Châtelier’s principle to predict the effect of
temperature changes.
However, heat is not matter and not written in a
proper equation.
76
The Effect of Temperature Changes on
Equilibrium for Exothermic Reactions
• For an exothermic reaction, heat is a product.
• Increasing the temperature is like adding heat.
• According to Le Châtelier’s principle, the equilibrium
will shift away from the added heat.
• The concentrations of C and D will decrease and the
concentrations of A and B will increase.
• The value of Keq will decrease.
• How will decreasing the temperature effect the system?
aA + bB cC + dD + heat
C D
K eq
a
b
A B
c
d
77
The Effect of Temperature Changes on
Equilibrium for Endothermic Reactions
• For an endothermic reaction, heat is a reactant.
• Increasing the temperature is like adding heat.
• According to Le Châtelier’s principle, the equilibrium
will shift away from the added heat.
• The concentrations of C and D will increase and the
concentrations of A and B will decrease.
• The value of Keq will increase.
• How will decreasing the temperature effect the system?
Heat + aA + bB cC + dD
Cc Dd
K eq
Aa Bb
78
The Effect of Temperature
Changes on Equilibrium
79
Practice—Predict the Effect on the Equilibrium
When the Temperature Is Reduced.
• Heat + 2 CO2(g) 2 CO(g) + O2(g)
• BaSO4(s) Ba2+(aq) + SO42-(aq) (endothermic)
• CH4(g) + 2 O2(g) CO2(g) + 2 H2O(l)
(exothermic)
80
Practice—Predict the Effect on the Equilibrium
When the Temperature Is Reduced
• Heat + 2 CO2(g) 2 CO(g) + O2(g)
Shift left, reducing the value of Keq.
• Heat + BaSO4(s) Ba2+(aq) + SO42-(aq)
Shift left, reducing the value of Keq.
• CH4(g) + 2 O2(g) CO2(g) + 2 H2O(l) + Heat
Shift right, increasing the value of Keq.
81
Solubility and Solubility Product
• Even “insoluble” salts dissolve somewhat in water.
Insoluble = less than 0.1 g per 100 g H2O.
• The solubility of insoluble salts is described in terms
of equilibrium between undissolved solid and
aqueous ions produced.
AnYm(s) n A+(aq) + m Y-(aq)
• Equilibrium constant for this process is called the
solubility product constant, Ksp.
Ksp = [A+]n[Y-]m
• If there is undissolved solid in equilibrium with the
solution, the solution is saturated.
• Larger Ksp = more soluble.
For salts that produce the same number of ions.
82
Example—Determine the Ksp of PbBr2 if its
Solubility Is 1.44 x 10-2 M.
PbBr2(s) Pb2+(aq) + 2 Br–(aq)
init -0
0
equil -0.0144
0.0288
Ksp = [Pb2+][Br–]2 = (0.0144)(0.0288)2 = 1.19 x 10-5
83
Example3.5—
Calculating Molar Solubility
from Ksp
84
Example 3.5:
• Calculate the molar solubility of BaSO4.
Ksp = 1.07 x 10-10 at 25 °C
85
Example 3.5:
Calculate the molar solubility
of BaSO4.
Ksp = 1.07 x 10-10 at 25 °C
Information:
Given: Ksp = 1.07 x 10-10
Find: [BaSO4], M = [Ba2+] = [SO42-]
Equation: Ksp = [Ba2+][SO42-]
Solution Map: Ksp → [Ba2+]
• Apply the solution map:
Ksp
Ba 2
Ba 2
1.07 10 10 Ba 2
1.03 10-5 Ba 2
1.07 10-10
SO4
2
Ba 2
2
2
Ba
90
Combustion as Redox
2 H2(g) + O2(g) 2 H2O(g)
92
Redox without Combustion
2 Na(s) + Cl2(g) 2 NaCl(s)
2 Na 2 Na+ + 2 e
Tro, Chemistry: A
Molecular Approach
93
Cl2 + 2 e 2 Cl
Reactions of Metals with Nonmetals
• consider the following reactions:
4 Na(s) + O2(g) → 2 Na2O(s)
2 Na(s) + Cl2(g) → 2 NaCl(s)
• the reaction involves a metal reacting with a nonmetal
• in addition, both reactions involve the conversion of
free elements into ions
4 Na(s) + O2(g) → 2 Na+2O– (s)
2 Na(s) + Cl2(g) → 2 Na+Cl–(s)
94
Oxidation and Reduction
• in order to convert a free element into an ion, the
atoms must gain or lose electrons
of course, if one atom loses electrons, another must
accept them
• reactions where electrons are transferred from one
atom to another are redox reactions
• atoms that lose electrons are being oxidized, atoms
that gain electrons are being reduced
Ger
Na+Cl–(s)
2 Na(s) + Cl2(g) → 2
Na → Na+ + 1 e– oxidation
Cl2 + 2 e– → 2 Cl– reduction
Leo
95
Electron Bookkeeping
• for reactions that are not metal + nonmetal, or do
not involve O2, we need a method for determining
how the electrons are transferred
• chemists assign a number to each element in a
reaction called an oxidation state that allows them
to determine the electron flow in the reaction
even though they look like them, oxidation states are
not ion charges!
oxidation states are imaginary charges assigned based on a
set of rules
ion charges are real, measurable charges
96
Rules for Assigning Oxidation States
• rules are in order of priority
1. free elements have an oxidation state = 0
Na = 0 and Cl2 = 0 in 2 Na(s) + Cl2(g)
2. monatomic ions have an oxidation state equal
to their charge
Na = +1 and Cl = -1 in NaCl
3. (a) the sum of the oxidation states of all the
atoms in a compound is 0
Na = +1 and Cl = -1 in NaCl, (+1) + (-1) = 0
97
Rules for Assigning Oxidation States
3. (b) the sum of the oxidation states of all the atoms in
a polyatomic ion equals the charge on the ion
N = +5 and O = -2 in NO3–, (+5) + 3(-2) = -1
4. (a) Group I metals have an oxidation state of +1 in all
their compounds
Na = +1 in NaCl
4. (b) Group II metals have an oxidation state of +2 in
all their compounds
Mg = +2 in MgCl2
98
Rules for Assigning Oxidation States
5. in their compounds, nonmetals have oxidation
states according to the table below
nonmetals higher on the table take priority
Nonmetal
Oxidation State
Example
F
-1
CF4
H
+1
CH4
O
-2
CO2
Group 7A
-1
CCl4
Group 6A
-2
CS2
Group 5A
-3
NH3
99
Practice – Assign an Oxidation State to
Each Element in the following
• Br2
• K+
• LiF
• CO2
• SO42-
• Na2O2
100
Practice – Assign an Oxidation State to
Each Element in the following
• Br2
Br = 0, (Rule 1)
• K+
K = +1, (Rule 2)
• LiF
Li = +1, (Rule 4a) & F = -1, (Rule 5)
• CO2
O = -2, (Rule 5) & C = +4, (Rule 3a)
• SO42-
O = -2, (Rule 5) & S = +6, (Rule 3b)
• Na2O2
Na = +1, (Rule 4a) & O = -1, (Rule 3a)
101
Oxidation and Reduction
Another Definition
• oxidation occurs when an atom’s oxidation state
increases during a reaction
• reduction occurs when an atom’s oxidation state
decreases during a reaction
CH4 + 2 O2 → CO2 + 2 H2O
-4 +1
0
+4 –2
+1 -2
oxidation
reduction
102
Oxidation–Reduction
• oxidation and reduction must occur simultaneously
if an atom loses electrons another atom must take them
• the reactant that reduces an element in another reactant
is called the reducing agent
the reducing agent contains the element that is oxidized
• the reactant that oxidizes an element in another reactant
is called the oxidizing agent
the oxidizing agent contains the element that is reduced
2 Na(s) + Cl2(g) → 2 Na+Cl–(s)
Na is oxidized, Cl is reduced
Na is the reducing agent, Cl2 is the oxidizing agent
103
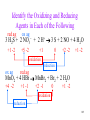
Identify the Oxidizing and Reducing
Agents in Each of the Following
3 H2S + 2 NO3– + 2 H+ 3 S + 2 NO + 4 H2O
MnO2 + 4 HBr MnBr2 + Br2 + 2 H2O
104
Identify the Oxidizing and Reducing
Agents in Each of the Following
red ag
ox ag
+1 -2
+5 -2
3 H2S + 2 NO3– + 2 H+ 3 S + 2 NO + 4 H2O
+1
0
+2 -2
+1 -2
oxidation
reduction
ox ag
red ag
+4 -2
+1 -1
MnO2 + 4 HBr MnBr2 + Br2 + 2 H2O
+2 -1
0
+1 -2
oxidation
reduction
105
Oxidation–Reduction Reactions
• We say that the element that loses electrons
in the reaction is oxidized.
• And the substance that gains electrons in the
reaction is reduced.
• You cannot have one without the other.
• In combustion, the O atoms in O2 are
reduced, and the non-O atoms in the other
material are oxidized.
106
Combustion as Redox
• In the following reaction:
2 Mg(s) + O2(g) 2 MgO(s)
• The magnesium atoms are oxidized.
Mg0 Mg2+ + 2 e
• The oxygen atoms are reduced.
O0 + 2 e O2
107
Combustion as Redox, Continued
• Even though the following reaction does not involve ion
formation, electrons are still transferred.
CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g)
• The carbon atoms are oxidized.
C4 C+4 + 8 e
These are not charges, they are called oxidation numbers, but
they help us see the electron transfer.
• The oxygen atoms are reduced.
O0 + 2 e O2
108
Bonding Theories
• explain how and why atoms attach together
• explain why some combinations of atoms are stable
and others are not
why is water H2O, not HO or H3O
• one of the simplest bonding theories was developed by
G.N. Lewis and is called Lewis Theory
• Lewis Theory emphasizes valence electrons to explain
bonding
• using Lewis Theory, we can draw models – called
Lewis structures – that allow us to predict many
properties of molecules
aka Electron Dot Structures
such as molecular shape, size, polarity
109
Why Do Atoms Bond?
• processes are spontaneous if they result in a system
with lower potential energy
• chemical bonds form because they lower the potential
energy between the charged particles that compose
atoms
• the potential energy between charged particles is
directly proportional to the product of the charges
• the potential energy between charged particles is
inversely proportional to the distance between the
charges
110
Potential Energy Between
Charged Particles
1 q1 q2
E potential
4 0 r
• 0 is a constant
= 8.85 x 10-12 C2/J∙m
• for charges with the same sign, Epotential is + and the
magnitude gets less positive as the particles get farther
apart
• for charges with the opposite signs, Epotential is and
the magnitude gets more negative as the particles get
closer together
• remember: the more negative the potential energy, the
more stable the system becomes
111
Potential Energy Between
Charged Particles
The attraction
repulsion between
like-charged particles
opposite-charged
increasesincreases
particles
as the as
particles
the
particles
get closer
get closer
together. To
Bringing
bring
them closer lowers
requiresthe
the addition
potential
energy
of more
of the
energy.
system.
112
Bonding
• a chemical bond forms when the potential
energy of the bonded atoms is less than the
potential energy of the separate atoms
• have to consider following interactions:
nucleus-to-nucleus repulsion
electron-to-electron repulsion
nucleus-to-electron attraction
113
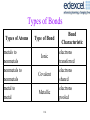
Types of Bonds
Types of Atoms
metals to
nonmetals
nonmetals to
nonmetals
metal to
metal
Type of Bond
Ionic
Covalent
Metallic
114
Bond
Characteristic
electrons
transferred
electrons
shared
electrons
pooled
Electronegativity
• measure of the pull an atom has on bonding
electrons
• increases across period (left to right) and
• decreases down group (top to bottom)
fluorine is the most electronegative element
francium is the least electronegative element
• the larger the difference in electronegativity,
the more polar the bond
negative end toward more electronegative atom
115
Electronegativity Scale
116
Electronegativity and Bond Polarity
• If difference in electronegativity between bonded atoms
is 0, the bond is pure covalent
equal sharing
• If difference in electronegativity between bonded atoms
is 0.1 to 0.4, the bond is nonpolar covalent
• If difference in electronegativity between bonded atoms
0.5 to 1.9, the bond is polar covalent
• If difference in electronegativity between bonded atoms
larger than or equal to 2.0, the bond is ionic
4%
0
0.4
Percent Ionic Character
51%
2.0
Electronegativity
117Difference
“100%”
4.0
Bond Polarity
ENCl = 3.0
3.0 - 3.0 = 0
Pure Covalent
Tro, Chemistry: A
Molecular Approach
ENCl = 3.0
ENH = 2.1
3.0 – 2.1 = 0.9
Polar Covalent
118
ENCl = 3.0
ENNa = 1.0
3.0 – 0.9 = 2.1
Ionic
119
Soaps are useful for cleaning because soap molecules have
both a hydrophilic end, which dissolves in water, as well
as a hydrophobic end, which is able to dissolve nonpolar
grease molecules. Although grease will normally adhere to
skin or clothing, the soap molecules can form micelles
which surround the grease particles and allow them to be
dissolved in water.
Tro's Introductory Chemistry, Chapter
15
120
Applied to a soiled surface, soapy water
effectively holds particles in colloidal
suspension so it can be rinsed off with
clean water. The hydrophobic portion
(made up of a long hydrocarbon chain)
dissolves dirt and oils, while the ionic
end dissolves in water. Therefore, it
allows water to remove normallyinsoluble matter by emulsification.
Sometimes the absence of oxygen in cold
and humid environment causes corpses to
naturally accumulate a soap-like coating,
adipocere, as covering the Soap Lady on
exhibit in the Mutter Museum.
121
Properties of Acids
• Sour taste.
• Change color of vegetable dyes.
• React with “active” metals, not noble
metals.
I.e., Al, Zn, Fe, but not Cu, Ag or Au.
Zn + 2 HCl ZnCl2 + H2
Corrosive.
• React with carbonates, producing CO2.
Marble, baking soda, chalk, limestone.
CaCO3 + 2 HCl CaCl2 + CO2 + H2O
• React with bases to form ionic salts.
And often water.
122
Common Acids
Chemical name
Formula
Old name
Strength
Nitric acid
HNO3
Aqua fortis
Strong
Sulfuric acid
H2SO4
Vitriolic acid
Strong
Hydrochloric acid
HCl
Muriatic acid
Strong
Phosphoric acid
H3PO4
Moderate
Chloric acid
HClO3
Moderate
Acetic acid
HC2H3O2
Hydrofluoric acid
HF
Carbonic acid
H2CO3
Boric acid
H3BO3
Vinegar
Weak
Weak
Soda water
Weak
Weak
123
Properties of Bases
•
•
•
•
A.k.a. alkalis.
Taste bitter.
Feel slippery.
Change color of vegetable dyes.
Different color than acid.
Litmus = blue.
• React with acids to form ionic salts.
And often water.
Neutralization.
124
Common Bases
Chemical
name
Sodium
hydroxide
Potassium
hydroxide
Calcium
hydroxide
Magnesium
hydroxide
Ammonium
hydroxide
Formula
Strength
KOH
Common
name
Lye,
caustic soda
Caustic potash
Ca(OH)2
Slaked lime
Strong
Mg(OH)2
Milk of magnesia
Weak
NH4OH, Ammonia water,
{NH3(aq)} aqueous ammonia
Weak
NaOH
Strong
Strong
125
Acid–Base Reactions
• Also called neutralization reactions because the acid
and base neutralize each other’s properties.
• In the reaction of an acid with a base, the H+1 from the
acid combines with the OH-1 from the base to make water.
• The cation from the base combines with the anion from
the acid to make the salt.
acid + base salt + water
2 HNO3(aq) + Ca(OH)2(aq) Ca(NO3)2(aq) + 2 H2O(l)
• The net ionic equation for an acid-base reaction often is:
H+1(aq) + OH-1(aq) H2O(l)
As long as the salt that forms is soluble in water.
126
Process for Predicting the Products of
an Acid–Base Reaction
1. Determine what ions each aqueous reactant has.
2. Exchange ions.
(+) ion from one reactant with (-) ion from the other.
H+ combines with OH− to make water.
3. Balance charges of combined ions to get formula of
the salt.
4. Balance the equation.
Count atoms.
5. Determine solubility of the salt.
Use the solubility rules.
If the salt is insoluble or slightly soluble, it will precipitate.
127