* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Vegetarian Food Panel: IgG
Cancer immunotherapy wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Gluten immunochemistry wikipedia , lookup
Immunocontraception wikipedia , lookup
IgA nephropathy wikipedia , lookup
Anaphylaxis wikipedia , lookup
Anti-nuclear antibody wikipedia , lookup
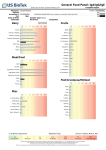
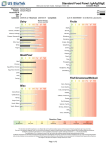
Vegetarian Food Panel: IgG Complete Report 16020 Linden Ave North, Shoreline WA 98133, USA Physician: Patient: Sample Report Accession #: Sex: F Age: 26 Date of Birth: Collected: Received: Dairy IgG CLIA #: 50D0965661 © US BioTek Laboratories Completed: Bovine-derived unless specified Grains/Legumes/Nuts Casein Almond Cheese, Cheddar Amaranth Cheese, Cottage Barley Cheese, Mozzarella Bean, Kidney Milk Bean, Lima Milk, Goat Bean, Pinto Whey Bean, Soy Yogurt Bean, String Buckwheat Cashew Nut Egg Coconut Corn Egg White, Chicken Flaxseed Egg Whole, Duck Gliadin, Wheat Egg Yolk, Chicken Gluten, Wheat Hazelnut Lentil Misc Oat Pea, Green Peanut Cocoa Bean Pecan Coffee Bean Pistachio Honey, Bee Rice, Brown Pepper, Black Rice, White Pepper, Chili Rye Sugar Cane Yeast, Baker's Sesame Seed Spelt Yeast, Brewer's Sunflower Seed Walnut, English Wheat, Whole Reaction Class This test does not identify anaphylaxis. Low allergen-IgE cannot justify secondary exposure to food suspect of inducing anaphylaxis as it may prove fatal. This test is not intended to diagnose, treat, cure, or prevent any disease or replace the medical advice and/or treatment obtained from a qualified healthcare practitioner. US BioTek's proprietary ELISA analysis is a semi-quantitative assessment for specific Total IgG (subclasses 1, 2, 3, 4) and IgE antibodies. The classification of 0 to VI denotes the level of IgG, IgA, and/or IgE antibodies detected through spectrophotometric analysis. US BioTek Laboratories, Inc. has developed and determined the performance characteristics of this test under the Clinical Laboratory Improvement Amendments (CLIA). This test has not been evaluated by the U.S. Food and Drug Administration and is considered for investigational and research purposes only. IgG and IgA antibodies may be associated with Delayed-Onset Hypersensitivity Reactions. IgE antibodies may be associated with Immediate-Onset Hypersensitivity Reactions. The antigens on the panel are subject to change without prior notice. Reference ranges are updated periodically. Vegetarian Food Panel: IgG Complete Report 16020 Linden Ave North, Shoreline WA 98133, USA Physician: Patient: Sample Report Accession #: Sex: F Age: 26 Date of Birth: Collected: Received: IgG CLIA #: 50D0965661 © US BioTek Laboratories Completed: Vegetables Fruits Artichoke Apple Avocado Apricot Bean Sprout Banana Bean, Navy Blueberry Beet Cantaloupe Broccoli Cherry Cabbage Cranberry Carrot Grape Cauliflower Grapefruit Celery Lemon Cucumber Orange Eggplant Papaya Garlic Peach Kamut Pear Lettuce Pineapple Millet Plum Mushroom Raspberry Olive Strawberry Onion Watermelon Pepper, Green Bell Potato, Sweet Potato, White Pumpkin Quinoa Radish Spinach Squash, Zucchini Tomato Reaction Class This test does not identify anaphylaxis. Low allergen-IgE cannot justify secondary exposure to food suspect of inducing anaphylaxis as it may prove fatal. This test is not intended to diagnose, treat, cure, or prevent any disease or replace the medical advice and/or treatment obtained from a qualified healthcare practitioner. US BioTek's proprietary ELISA analysis is a semi-quantitative assessment for specific Total IgG (subclasses 1, 2, 3, 4) and IgE antibodies. The classification of 0 to VI denotes the level of IgG, IgA, and/or IgE antibodies detected through spectrophotometric analysis. US BioTek Laboratories, Inc. has developed and determined the performance characteristics of this test under the Clinical Laboratory Improvement Amendments (CLIA). This test has not been evaluated by the U.S. Food and Drug Administration and is considered for investigational and research purposes only. IgG and IgA antibodies may be associated with Delayed-Onset Hypersensitivity Reactions. IgE antibodies may be associated with Immediate-Onset Hypersensitivity Reactions. The antigens on the panel are subject to change without prior notice. Reference ranges are updated periodically.