* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download class notes packet - Social Circle City Schools
Depletion force wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Stöber process wikipedia , lookup
Condensed matter physics wikipedia , lookup
Spinodal decomposition wikipedia , lookup
Wave–particle duality wikipedia , lookup
Vapor–liquid equilibrium wikipedia , lookup
Matter wave wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Freeze-casting wikipedia , lookup
Colloidal crystal wikipedia , lookup
Sol–gel process wikipedia , lookup
Degenerate matter wikipedia , lookup
Elementary particle wikipedia , lookup
Particle-size distribution wikipedia , lookup
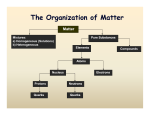
Matter Main Idea Chemistry Details Chemistry is Launch Lab Matter Mass and Volume CHEM IS TRY! Matter is anything that has a __________ and takes up ___________ (Volume) Mass : amount of ________ in an object. Measures in _______ using a _____________ Identifying matter Density Volume: amount of _________an object takes up Measured in ______ or _____ Using a _______ _____ or a ________________ __________ What is one of the most reliable ways to describe matter? Answer: __________________ Which picture is more dense? Why? Annotate Definition of density: ____________ per given unit of ___________ Break down the definition Mass per given unit of volume Vocab words ( box out) New definition?____________________________________________ It means: How much_________________________________________ Equation D= _______________ M= _________ V= _________________ Make it easy Units? How would you? How about that guy? Displacement Think it through What do you thing the units will be? ________________ M units/ (_____) / V units (_____) Do they cancel? Units are : _____________ or __________________ How? What equipment? (hint think about the units) What equipment? Which is right ? A or B Displacement is : how much space an object ___________ another away. We can use the displacement of ________________ in a graduated cylinder to figure out the _____________ of an object that is not square. Draw a model! Keep thinking Label 1-5 Most Least Practice worksheets Apply. Classifying Matter Write a procedure. Are water and Kool- aid the same? Why? Why not? How about metal and air? Why? Why not? Matter needs ____________________ or a ____________________ system Graphic organizer Pure Substances Elements Pure substances are subdivided into two categories ____________ and _________________. An _______________ is a substance that __________________ be _______________ into simplier substances by chemical reactions. In the __________________ table there are over ______ of these. Most of them can be found _____________ on Earth. Others are produced _____________________ by ___________________ reactions Describe what you see Symbols of Elements _________________ are indicated by symbols. The _________ letter of the symbol of and element is always _________________________. The ____________ letter is never ______________________. Examples: Compounds A _______________________ is a substance that is composed of two or more ___________________ _____________________ held together in _________proportions A ___________________ for a compund identifies the ___________________ and the kind of _________________ in a compund and the proportion in which they are found. Examples: The number at the __________________ of a symbol is called a ___________________. The _________________ at the bottom of symbol indicates the number of ___________. ________________ are the building block of ___________________ In the formula for bleach how many Ca? O? Cl? How did you figure that out? ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ ____________ Mixtures A _________________________ is a combination of _____ or more substances in which each substances’ _____________________ is ____________________. The mixture ____________________ have uniform composition The substance in a mixture can be separated by ________________ means. Homogeneous Mixtures Parts to a solution Heterogeneous Mixture What does this mean in your own words? ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ ________ _________________________ mixtures are __________________ which have a _________________ composition and appearance throughout. A _______________ may be _______________(air), ______________ (brass) or liquid (___________________) An _____________ is a solution of two ____________. Solutions have ________ parts __________________- the substance that is dissoved, present in smaller amounts __________________-the substance that does the __________________, present in larger amount. Compare and contrast. How are they the same? ______________________________________________________________ ______________________________________________________________ ____ How are they different? ______________________________________________________________ ______________________________________________________________ ____ Solution? Why? ______________________________________________________________ ______________________________________________________________ ____ _______________________mixtures are mixtures that do ______ have _____________ composition and appearance throughout The individual components which make up the mixture remain ___________________separated and can be seen as separate components. In your own words? ______________________________________________________________ ______________________________________________________________ __There are _______ types of heterogeneous mixtures. ________________ and ______________________. Examples: Characteristics of Fill in the chart. Mixtures Type of mixture Tyndall Effect Concept Check Separating a Mixture Real talk: BP oil spill Filtration Size of particles Settles out Tyndall Effect A ____________________ Tyndall effect is the ____________ in the beaker are ____________ enough to scatter light. A ____________________Tyndall effect you cannot see the _________ of light. There are no particles to _______________ the beam of light. D. E. E. D. S ON separate paper to turn in! ___________ways to separate a mixture 1. __________________________ 2. __________________________ 3. __________________________ 4. __________________________ Reading for meaning for a grade Suggestions? LAB- report due for a grade Filtration separates a _____________ from a _________ through the use of a porous material as a _______________. Paper, charcoal or sand can serve as a filter. The ___________ can pass through but not the _________. When can this be used? Distillation Distillation is a method used to separate a mixture of __________________ according to their ____________ points. When can this be used? Chromatography Chromatography is a method for analyzing complex _______________ such as _____ by separating them into the chemicals fro which they are made. Attraction to a medium will take light particles up the medium as heavier particles stay low on the medium When can this be used? Crystallization Crystallization refers to the formation of solid crystals from a _________________________ solution. It is essentially a ________ __________ separation technique. Lab When can this be used? Is that water safe? States of Matter and Phase changes Write..Chalk Talk Solids Liquids Gases Plasmas Time to check out the water to see how it relates to matter! ____ states of matter. Match words with pictures. Answer? How did you decide which was which? ______________________________________________________________ _____________________________________________________________ What did you notice about the difference between your choices? ______________________________________________________________ ______________________________________________________________ What makes the particles stay close or move apart? ______________________________________________________________ ______________________________________________________________ Solids are __________________packed particles Definite _______________ (lxwxh) Definite ________________ ____________________- through the _____________ method ______________ of particles. Liquids are __________________packed particles ________________ volume (___________________ cylinder) Definite _________ take the shape of the ________________ Density ______ and ___________ of particles. Gases are No _____________________ of particles No _______________ _volume ( must be ________________) No definite ____________- can be contained Density- only if in a _____________ minus the mass of the container ___________________ motion Plasmas are No organization of particles No definite _____________ No definite _____________ No density because we _________ catch it __________ energy - ______________ motion ENERGY Compare and contrast All states of matter can change because of energy! solid energy liquid energy Changes gas energy If I am moving slow and start moving faster am I incresing or decreasing my energy? Answer: Draw a diagram that puts states of matter in order from low to high energy Increasing energy Place the words in between states of matter where the process would occur Boiling Evaporation/ vaporization Melting Sublimation Freezing Deposition Condensation ______________ is from __________ to ____________ Example: Evaporization / boiling / vaporization is from ________________ to ______ Example: _____________ is a solid to a _____________(no liquid phase) Example: Decreasing _____________________ is a ____ to ________________ energy Example: Freezing is ____________ to a ______________ Example: Deposition is a ___________ to a ____________ (no liquid phase) Example: Mind map THE END For a grade Good luck on your test!