* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Unit5C - OCCC.edu
Electrical resistivity and conductivity wikipedia , lookup
Transition state theory wikipedia , lookup
Citric acid cycle wikipedia , lookup
Chemical bond wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Click chemistry wikipedia , lookup
Electron configuration wikipedia , lookup
Atomic theory wikipedia , lookup
Stoichiometry wikipedia , lookup
Electrolysis of water wikipedia , lookup
Marcus theory wikipedia , lookup
Acid–base reaction wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Flux (metallurgy) wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Microbial metabolism wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Water splitting wikipedia , lookup
Coordination complex wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Surface properties of transition metal oxides wikipedia , lookup
Metallic bonding wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Geochemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
Extended periodic table wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Oxidation state wikipedia , lookup
Electrochemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
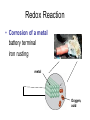
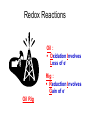
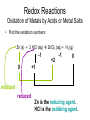
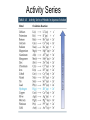
Recap Precipitation Reactions: ions combine to form insoluble products Neutralization Reactions: H+ ions and OH- ions combine to form H2O Next: Oxidation-Reduction (Redox) Reactions: Atoms or ions donate or accept electrons Redox Reaction • Corrosion of a metal battery terminal iron rusting metal Oxygen, acid Metal Corrosion • metal loses electron forms a cation = metal is oxidized AND another reactant gains electrons = is reduced Oxidation-Reduction Reactions • An oxidation occurs when an atom or ion loses electrons. • A reduction occurs when an atom or ion gains electrons. • One cannot occur without the other. Redox Reactions • Oxidation-Reduction Reactions (Redox Reactions) – reactions that involve the transfer of electrons between two reactants – an element in one reactant is oxidized while an element in another reactant is reduced Mg (s) + 2 H+ (aq) Mg2+ (aq) + H2 (g) oxidized reduced Redox Reactions • Oxidation: – the loss of electrons • chemical species becomes more positively charged – the gain of oxygen – the loss of hydrogen Redox Reactions • Reduction: – the gain of electrons • the chemical species becomes more negatively charged – the gain of hydrogen – the loss of oxygen Redox Reactions GER LEO LEO says GER • LEO: – Lose Electrons Oxidation • GER: – Gain Electrons Reduction Redox Reactions Oil : Oxidation Involves Loss of eRig : Reduction Involves Gain of eOil Rig Redox Reactions • Electrons are not explicitly shown in chemical equations. • Oxidation Numbers are used to keep track of electrons gained and lost during redox reactions. • Oxidation number – a hypothetical number assigned to an individual atom present in a compound using a set of rules. • May be positive, negative, or zero Rules for Oxidation Numbers • Oxidation numbers are always reported for individual atoms or ions not groups of atoms or ions!!!!!!!!!!! • For an atom in its elemental form, the oxidation number is always zero. – H2: oxidation # = 0 for each H atom – Cu: oxidation number = 0 – Cl2: oxidation # = 0 for each Cl atom Rules for Oxidation Numbers • For any monoatomic ion: oxidation # = charge on ion • K+ oxidation # = +1 • Cl- oxidation # = -1 • S2- oxidation # = -2 Rules for Oxidation Numbers • Group 1A Metal Cations: Always +1 • Group 2A Metal Cations: Always +2 • Hydrogen (H) +1 when bonded to nonmetals -1 when bonded to metals Rules for Oxidation Numbers • Oxygen (O) -1 in peroxides (O22-) -2 in all other compounds • Fluorine (F) always -1 Rules for Oxidation Numbers • The sum of the oxidation numbers of all atoms in any chemical species (ion or neutral compound) is equal to the charge on that chemical species H2O: MgCl2: 1 + 1 + (-2) = 0 2 + (-1) + (-1) = 0 MnO4-: 7 + (-2) + (-2) + (-2) + (-2) = -1 Oxidation Numbers • For many compounds, you will be able to directly apply the rules to determine the oxidation number of all atoms except for one. – Use the last two rules to determine the oxidation number of that last element. Practice determining oxidation numbers Example: Determine the oxidation state of all elements in SO3. Is it elemental? No Are any monoatomic ions present? Which elements have rules? Set up an equation to find the remaining oxidation number. S + 3(-2) = 0 No O = -2 S = +6 Oxidation Numbers Example: Determine the oxidation number of Mn and O in MnO4-. Is it elemental? No Are any monoatomic ions present? Which elements have rules? No O = -2 Set up an equation to find the remaining oxidation number. Mn + 4(-2) = -1 so Mn = +7 Oxidation Numbers Example: Determine the oxidation state of all elements in NaNO3 Is it elemental? No Na+ Are any monoatomic ions present? Which elements have rules? Set up an equation to find the remaining oxidation number. 1 + N + 3(-2) = 0 Na = +1, O = -2 N = +5 Practice 1) Determine the oxidation number of P in HPO42-. 2) Determine the oxidation state of all elements in Cr2O72-. 3) Determine the oxidation state of Sn in SnBr4. Redox Reactions • There are many different kinds of redox reactions. – Combustion CH4 (g) + 2 O2 (g) CO2 (g) + 2 H2O (g) – Oxidation of Metals by Acids or Metal Salts Cu (s) + H2SO4 (aq) CuSO4 (aq) + H2 (g) Redox Reactions Oxidation of Metals by Acids or Metal Salts • The reaction between a metal and an acid or between a metal and a metal salt is called a displacement reaction. – a reaction in which an element reacts with a compound and displaces an element from that compound A + BX AX + B Redox Reactions Oxidation of Metals by Acids or Metal Salts Examples: Mg (s) + 2HCl (aq) MgCl2 (aq) + H2 (g) Zn (s) + 2 HBr (aq) ZnBr2 (aq) + H2 (g) Mn (s) + Pb(NO3)2 (aq) Mn(NO3)2 (aq) + Pb (s) Redox Reactions Oxidation of Metals by Acids or Metal Salts • How do you know if a redox reaction has occurred? • You must examine the oxidation number of each of the elements present in the reactants and products. – If the oxidation number changes, then a redox reaction has occurred. Redox Reactions Oxidation of Metals by Acids or Metal Salts • When oxidation occurs: – Electrons are lost – Oxidation number increases e- • When reduction occurs: – Electrons are gained – Oxidation number is reduced (decreases) e- Redox Reactions Oxidation of Metals by Acids or Metal Salts Mg(s) + 2HCl (aq) MgCl2 (aq) + H2 (g) -1 0 +1 Oxidation # of Mg increased oxidation Oxidation # of H+ ion reduced reduction -1 +2 0 Redox Reactions Oxidation of Metals by Acids or Metal Salts Example: Identify the element that has been oxidized and the one that has been reduced. Zn (s) + 2 HCl (aq) ZnCl2 (aq) + H2 (g) Redox Reactions Oxidation of Metals by Acids or Metal Salts • Find the oxidation numbers: Zn (s) + 2 HCl (aq) ZnCl2 (aq) + H2 (g) -1 0 +2 -1 0 +1 oxidized reduced Zn is the reducing agent. HCl is the oxidizing agent. Redox Reactions Oxidation of Metals by Acids or Metal Salts • Oxidizing agent: – The reactant that causes another reactant to be oxidized – The reactant that contains the element that was reduced • Reducing agent: – The reactant that causes another reactant to be reduced – The reactant that contains the element that was oxidized. Redox Reactions Oxidation of Metals by Acids or Metal Salts Example: Identify the oxidizing and reducing agents in the following reaction. CH4 (g) + 2 O2 (g) CO2 (g) + 2 H2O (g) Redox Reactions Oxidation of Metals by Acids or Metal Salts • You can also write a net ionic equation to describe a redox reaction: – Write all soluble strong electrolytes as ions – Eliminate the spectator ions Zn (s) + 2 HCl (aq) ZnCl2 (aq) + H2 (g) Zn (s) + 2 H+ (aq) + 2 Cl- (aq) Zn2+ (aq) + 2 Cl- (aq) + H2 (g) Zn (s) + 2H+ (aq) Zn2+ (aq) + H2 (g) Redox Reactions Oxidation of Metals by Acids or Metal Salts Example: Write the complete ionic and net ionic equations for the reaction. Which element is oxidized? What is the oxidizing agent? 2 Al (s) + 3 Ni(NO3)2 (aq) 2 Al(NO3)3 (aq) + 3 Ni (s) 2 Al (s) + 3 Ni2+(aq) + 6 NO3- (aq) 2 Al3+ (aq) + 6 NO3- (aq) + 3 Ni (s) 2 Al (s) + 3 Ni 2+(aq) 2 Al3+ (aq) + 3 Ni (s) Redox Reactions Oxidation of Metals by Acids or Metal Salts +5 +5 2 Al (s) + 3 Ni(NO3)2 (aq) 2 Al(NO3)3 (aq) + 3 Ni (s) 0 oxidized +2 -2 +3 -2 0 Redox Reactions Oxidation of Metals by Acids or Metal Salts • Based on the previous equation, we wouldn’t want to store a solution of Ni(NO3)3 in an aluminum container. – The aluminum container would react and dissolve!!! • Metals differ in the ease with which they are oxidized. – Al(s) is oxidized by Ni(NO3)3 (aq) – Ag(s) is NOT oxidized by Ni(NO3)3 (aq) Redox Reactions Oxidation of Metals by Acids or Metal Salts • Activity series: – A list of metals arranged in order of decreasing ease of oxidation – Used to predict whether a metal will react with an acid or with a metal salt – See Table 4.5 – Any metal on the list can be oxidized by, i.e. will lose electrons to, the ions of a metal below it. Activity Series Activity series: Any metal on the list can be oxidized by, i.e. will lose electrons to, the ions of a metal below it. Cu can lose electrons to Ag+