* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download O 2
Pre-Bötzinger complex wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Freediving blackout wikipedia , lookup
High-altitude adaptation in humans wikipedia , lookup
Alveolar macrophage wikipedia , lookup
Organisms at high altitude wikipedia , lookup
Intracranial pressure wikipedia , lookup
Cardiac output wikipedia , lookup
Acute respiratory distress syndrome wikipedia , lookup
Hemodynamics wikipedia , lookup
Physiology of decompression wikipedia , lookup
Biofluid dynamics wikipedia , lookup
Circulatory system wikipedia , lookup
Homeostasis wikipedia , lookup
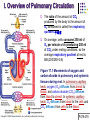
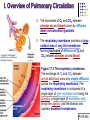
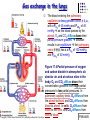
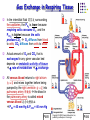
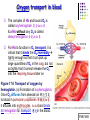
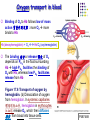
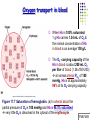
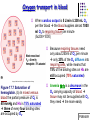
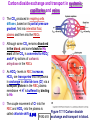
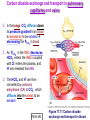
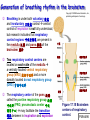
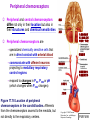
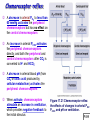
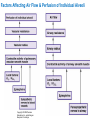
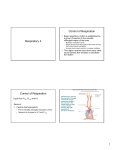
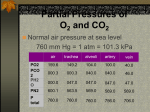
Chapter 17 The Respiratory System: Gas Exchange and Regulation of Breathing 1. Overview of the pulmonary circulation 肺循環總論 2. Diffusion of gases 氣體的擴散 3. Exchange of oxygen and carbon dioxide 氧氣及二氧化碳的交換 4. Transport of gases in the blood 氣體在血液的運送 5. Central regulation of ventilation 中樞調控換氣 6. Control of ventilation by chemoreceptors 化學接受器調控換氣 7. Local regulation of ventilation and perfusion 換氣及灌流的局部調控 8. The respiratory system in acid-base homeostasis 呼吸系統在酸鹼恆定的作用 I. Overview of Pulmonary Circulation The ratio of the amount of CO2 produced by the body to the amount of O2 consumed is called the respiratory quotient 呼吸商 On average, cells consume 250 ml of O2 per minute while producing 200 ml of CO2 under resting conditions, so the average respiratory quotient at rest is 0.8 (200/250=0.8) Figure 17.1 Movements of oxygen and carbon dioxide in pulmonary and systemic tissues during rest. In pulmonary capillary beds, oxygen (O2) diffuses from alveoli to blood, and carbon dioxide (CO2) diffuses from blood to alveoli. In systemic capillary beds, O2 diffuses from blood to the cells and CO2 diffuses from cells to the blood. Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings. P478-479 I. Overview of Pulmonary Circulation The movement of O2 and CO2 between alveolar air and blood occurs by diffusion down concentration gradients Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings. The respiratory membrane provides a large surface area of very thin membrane, favoring fast rates of diffusion of O2 and CO2 between alveolar air and blood Figure 17.2 The respiratory membrane. The exchange of O2 and CO2 between alveoli and blood occurs by simple diffusion across the respiratory membrane. The respiratory membrane is composed of a single layer of type I epithelial cells lining the alveolus, a single layer of endothelial cells lining the capillary, and the alveolar and capillary basement membranes. P479-480 II. Diffusion of Gases Partial pressure of gases According to the ideal gas law 理想氣體定律, for any pure gas, PV=nRT, where P = pressure, V = volume, n = number of moles, R = universal gas constant, and T = temperature If V and T are constant, then the pressure exerted by a gas is directly proportional to the number of moles of the gas A gas (air, for instance) is often a mixture of more than one type of molecule the total pressure of such a gas is the sum of the pressures of individual gases that make up the mixture: Ptotal = P1 + P2 + P3 + … Pn n: number of gases Dalton’s law 道耳吞定律 states that the pressure exerted by the individual gases occupying the same volume alone the pressure exerted by an individual gas in a mixture is called the partial pressure 分壓 of that gas P480 Partial pressure of gases Composition of Air 79% nitrogen (N2) + 21% oxygen (O2) + trace amounts carbon dioxide (CO2), helium, argon, etc. Water (H2O) can be a factor depending on humidity 溼度 Pair = 760 mm Hg = PN2 + PO2 PN2 = 0.79 x 760 mm Hg = 600 mm Hg PO2 = 0.21 x 760 mm Hg = 160 mm Hg Air is only 0.03% carbon dioxide PCO2 = 0.0003 x 760 mm Hg = 0.23 mm Hg Pair = 760 mm Hg = PN2 + PO2+ PH2O (when100% humidity) PN2 = 0.741 x 760 mm Hg = 563 mm Hg PO2 = 0.196 x 760 mm Hg = 149 mm Hg PH2O = 0.062 x 760 mm Hg = 47 mm Hg PCO2 = 0.00027 x 760 mm Hg = 0.21 mm Hg P480-481 Solubility of gases in liquids The ability of a gas to dissolve in water has physiological significance because O2 and CO2 are exchanged between air in the alveoli and blood (which is primarily water) and are then transported by the blood The concentration of dissolved gas molecules depends not only on the partial pressure 分壓 but also on the solubility 溶解度 of the gas in that particular liquid Henry’s law 亨利定律 Henry’s law states that when the temperature 溫度 is constant, the concentration 濃度 of a gas in a liquid is proportional to its partial pressure c = kP c is the molar concentration 莫爾濃度 of the dissolved gas (moles/liter), P is the partial pressure 分壓 of the gas, and k is the Henry’s law constant 常數 at a specific temperature Because k is a constant, if the pressure changes, the relationship between the concentration of dissolved gas 溶解的氣體 at the initial pressure (P1) and at the subsequent pressure (P2) can be described by c1/P1 = c2/P2 Therefore, the concentration of a gas in plasma is directly related to the partial pressure of the gas, and movement of a gas by diffusion occurs from areas of high partial pressure to areas of low partial pressure P481 Solubility of gases in liquids Figure 17.3 Solubilities of oxygen and carbon dioxide in water. The beakers at left depict the initial conditions in which water has just been exposed to air that has a partial pressure of gas of 100 mmHg; the breakers at right show the conditions after equilibration, when the gas has dissolved in the water until the gas’s partial pressure in both media are equal. (a) After equilibration, the concentration of O2 in water is much less than its concentration in air, indicating that the solubility of O2 in water is low. (b) After equilibration, the concentration of CO2 in water is greater than that of O2 in water at the same partial pressure, indicating that CO2 is more soluble in water than is O2. Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings. P483 III. Exchange of Oxygen and Carbon Dioxide Gas exchange in the lungs The exchange of O2 and CO2 between the alveoli and the blood, and between the blood and systemic tissues, occurs by the same mechanism each gas diffuses down its own partial pressure gradient In atmospheric air, the PO2 is 160 mmHg, and the PCO2 is 0.23 mmHg however, the PO2 in the alveoli is only 100 mmHg, whereas the PCO2 is 40 mmHg Alveolar gas pressure differ from atmospheric pressures for three reasons: exchange of gases occurs continually between alveolar and capillary blood upon inspiration 吸氣, fresh atmospheric air mixes with air rich in CO2 are relatively poor in O2 in the dead space of the conducting zone air in the alveoli is saturated 飽和 with water vapor P483 Gas exchange in the lungs The blood entering the pulmonary capillaries is deoxygenated blood 去氧血, with a PO2 of 40 mmHg and PCO2 of 46 mmHg as this blood passes by the alveoli, O2 and CO2 diffuse down their partial pressure gradient diffusion results in an equilibrium the pulmonary veins 肺靜脈 has a a PO2 of 100 mmHg and PCO2 of 40 mmHg Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings. Figure 17.4 Partial pressure of oxygen and carbon dioxide in atmospheric air, alveolar air, and at various sites in the body. O2 and CO2 diffuse down their concentration gradients from high partial pressures to low partial pressures. In pulmonary capillary beds, O2 diffuses from the alveoli to blood, and CO2 diffuses from blood to alveoli. In cells, O2 diffuses from blood to the cells, and CO2 diffuses from cells to blood. P484-485 Gas exchange in the lungs Diffusion is a rapid process provides a margin of safety 安全範圍 in that gases can still equilibrate between capillary blood and alveolar air even if blood is flowing at a rate up to three times faster than the resting rate, as can occur during exercise The diffusion rate is rapid because of the thinness of the respiratory membrane 呼吸性的膜很薄 whenever the respiratory membrane is effectively thickened 變厚, gas exchange is hampered 阻礙, such as pulmonary edema 肺水腫 Figure 17.5 Gas exchange as a function of pulmonary capillary length. In the rapid exchange of gases between alveolar air and pulmonary capillary blood, equilibration of the partial pressure of (a) O2 and (b) CO2 occurs within the first 33% of a capillary’s length. Mixed venous blood refers to the blood in the pulmonary artery 肺動脈 that contains blood returned to the heart from the systemic veins 體靜脈. Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings. P485 Gas Exchange in Respiring Tissue In the interstitial fluid 間質液 surrounding the capillaries, the PO2 is lower because respiring cells consume O2 , and the PCO2 is higher because the cells produce CO2 O2 diffuses from blood to cells; CO2 diffuses from cells to blood Actual amount of O2 and CO2 that is exchanged in any given vascular bed depends on metabolic activity of tissue rate of metabolism exchange All venous blood returns to right atrium 右心房 and mixes together before being pumped by the right ventricle 右心室 into pulmonary artery 肺動脈 the blood in the pulmonary artery is called mixed venous blood 混合的靜脈血 PO2 = 40 mm Hg & PCO2 = 46 mm Hg P485 Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings. P484 Determinants of Alveolar PO2 and PCO2 Factors affecting alveolar partial pressures PO2 and PCO2 of inspired air Minute alveolar ventilation Rates at which respiring tissue consume O2 and produce CO2 Most critical is rate of alveolar ventilation relative to rate of O2 consume and CO2 production Hyperpnea 呼吸增強 = increased ventilation due to increased demand minimal changes in arterial PO2 and PCO2 Hypoventilation 換氣不足 = ventilation does not meet demands arterial PO2 decreases & arterial PCO2 increases Hyperventilation 換氣過度 = ventilation exceeds demands arterial PO2 increases & arterial PCO2 decreases P486 Table 17.2 Some term used in respiratory physiology 呼吸增強 呼吸困難 呼吸暫停 呼吸過快 換氣過度 換氣不足 氧氣不足 低血氧 高血碳酸 低血碳酸 Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings. P487 IV. Transport of Gases in Blood Oxygen transport in blood Transport of O2 in the blood has a special need the transport mechanism must be readily 容易地 reversible 可逆的 hemoglobin (Hb) 血紅素 has a unique structure that allows O2 to do just that Every liter of arterial blood contains about 200 ml of O2 O2 not very soluble in plasma thus only 3.0 mL/200 ml arterial blood O2 dissolved in plasma (1.5%) & other 197 mL of O2 (98.5%) is transported by Hb 以血紅素運送 Hb contains of four subunits 4 globins 球蛋白 (2 alpha & 2 beta) 4 heme 血基質 groups that contain iron (Fe2+) 鐵離子 O2 binds to heme group 4 heme groups per Hb thus each Hb can bind 4 O2 P487 Copyright © 2005 Pearson Education, Inc., publishing as Benjamin Cummings. Oxygen transport in blood The complex of Hb and bound O2 is called oxyhemoglobin 氧合血紅素 & a Hb without any O2 is called deoxyhemoglobin 去氧血紅素 For Hb to function in O2 transport, it is critical that it binds the O2 reversibly tightly enough so that it can pick up large quantities of O2 in the lung, but not so tightly that it cannot release the O2 into the respiring tissues later on Figure 17.6 Transport of oxygen by hemoglobin. (a) Formation of oxyhemoglobin. Once O2 diffuses from alveolar air 肺泡空氣 to blood in pulmonary capillaries 肺臟微血管, it diffuses into erythrocytes 紅血球and binds to hemoglobin for transport 運送in the blood. P487-488 Oxygen transport in blood Binding of O2 to Hb follows law of mass action 質量作用定律 : more O2 more binds to Hb Hb (deoxyhemoglobin) + O2 Hb.O2 (oxyhemoglobin) The binding 結合 or release 釋放 of O2 depends on PO2 in the fluid surrounding Hb high PO2 facilities the binding of O2 with Hb, whereas low PO2 facilitates release from Hb Figure 17.6 Transport of oxygen by hemoglobin. (b) Dissociation of oxygen from hemoglobin. In systemic capillaries 體循環微血管, hemoglobin in erythrocytes 紅血球 release O2, which then diffuses 擴散 from blood into tissue cells. P487-488 Oxygen transport in blood When Hb is 100% saturated, 1 g Hb carries 1.34 mL of O2 & the normal concentration of Hb in blood is an average 150 g/L The O2 -carrying capacity of the Hb in blood is about 200 mL O2 per liter of blood (1.34x150=200) at normal arterial PO2 of 100 mmHg, Hb is at approximately 98% of its O2-carrying capacity Figure 17.7 Saturation of hemoglobin. (a) In arterial blood the partial pressure of O2 is 100 mmHg and Hb is 98.5% saturated very little O2 is dissolved in the cytosol of the erythrocyte. P487-489 Oxygen transport in blood When cardiac output is 5 L/min & 200 mL O2 per liter blood the blood supplies almost 1000 ml O2 to respiring tissue per minute (5x200=1000) Because respiring tissues need only about 250ml of O2 per minute only 25% of the O2 diffuses into respiring cells, while means that 75% of the binding sites on Hb are still occupied (75% saturated) Figure 17.7 Saturation of hemoglobin. (b) In mixed venous blood the partial pressure of O2 is 40 mmHg and Hb is 75% saturated three of every four binding sites are occupied by O2. Anemia 貧血 is a decrease in the O2 carrying capacity of blood tissue may not be supplied with O2 they need tire more easily P488-489 The hemoglobin-oxygen dissociation curve Figure 17.8 Hemoglobin-oxygen dissociation curve. The binding of O2 to Hb depends on the partial pressure of O2 in blood. At low partial pressures, little O2 is bound to Hb. As PO2 increase, binding increases and then levels off as saturation approaches 100%. The binding of O2 to Hb is non-linear 非線性 S-shaped (sigmoidal) 乙狀 The binding of one O2 to Hb increases the affinity 增加親和力 of the Hb for O2 and therefore increases the likelihood that another O2 will bind with Hb positive cooperatively 正的協同作用 Copyright © 2005 Pearson Education, Inc., publishing as Benjamin Cummings. P489 Other factors affecting the affinity of hemoglobin for oxygen Temperature, pH, PCO2, 2,3-DPG At least four other factors (temperature, pH, PCO2, and 2,3-DPG) affect the affinity of Hb for O2 The first three factors ─ temperature, pH, and PCO2─all work to promote O2 unloading 卸下 from Hb in respiring tissues and O2 loading 裝載 in the lungs, both of which increase the efficiency of O2 exchange at and transport to the tissues that need it 2,3-DPG (2,3-diphosphoglycerate) is produced in erythrocytes from an intermediate compound 中間物 in anaerobic 無氧呼吸 glycolysis 醣解作用 under conditions of low O2 such as anemia 貧血 and high altitude 高海拔 Synthesis of 2,3-DPG is inhibited by oxyhemoglobin 氧合血紅素 & 2,3-DPG decreases affinity 降低親和力 of Hb for O2 enhancing O2 unloading 卸下 Another factor that affects the binding of O2 to Hb is carbon monoxide (CO) 一氧化碳 CO is toxic because when present it binds to Hb more readily than O2, which prevents O2 from binding and decreases the O2 -carrying capacity of blood P490-491 Other factors affecting the affinity of hemoglobin for oxygen Changes of affinity Rightward shift 右移 (親和力降低) Higher PO2 necessary to saturate Hb (harder loading) 需要較高的氧分壓才能使血紅素飽和,較 不易裝載 Easier unloading of O2 較容易卸下 Leftward shift 左移 (親和力增加) Easier loading 容易裝載 Harder unloading 不容易卸下 Figure 17.9 Effects of changes in the affinity of hemoglobin for oxygen on the hemoglobin-oxygen dissociation curve. An increase in affinity of Hb for O2 causes a leftward shift 左移 in the curve, whereas a decrease in affinity of Hb for O2 causes a rightward shift 右移 in the curve. P490 Other factors affecting the affinity of hemoglobin for oxygen Temperature Temperature 溫度 affects the affinity of Hb for O2 by altering the structure of the Hb molecule As metabolic of tissues increases, temperature increases decrease Hb affinity for O2 increases O2 unloading in tissue Figure 17.10 Effects of temperature and pH on the hemoglobin-oxygen dissociation curve. (a) Increases or decreases in temperature 溫度 from normal body temperature (370C) cause decreases or increases, respectively, in the affinity of Hb for O2. P490 Other factors affecting the affinity of hemoglobin for oxygen pH The effect of pH on Hb-O2 dissociation curve is known as the Bohr effect 伯爾氏效應 when O2 binds to Hb, certain amino acids in the protein release hydrogen ions (H+) Hb + O2 Hb.O 2 + H+ Law of mass action Active tissue produces H+ (a decrease in pH ) push the reaction to left decreases affinity (rightward shift) increases O2 unloading Figure 17.10 Effects of temperature and pH on the hemoglobin-oxygen dissociation curve. (b) Increases or decreases in pH 酸鹼值 from normal arterial pH (7.4) cause increases or decreases, respectively, in the affinity of Hb for O2. P490-491 Other factors affecting the affinity of hemoglobin for oxygen PCO2 The PCO2 affects the affinity of Hb for O2 because carbon dioxide (CO2) reacts reversibly with certain amino groups in the Hb to form carbaminohemoglobin (HbCO2) 碳醯胺基血紅素 Hb + CO2 HbCO2 Increased metabolic activity increases CO2 ( PCO2) pushes the reaction to the right HbCO2 changes Hb’s conformation and decreases its affinity for O2 increases O2 unloading in active tissue carbamino effect 碳醯胺基效應 P491 Carbon dioxide transport in the blood Of all the CO2 in the blood, 5-6% is dissolved, 5-8% is bound to Hb as HbCO2, and 86-90% is dissolved in the blood as bicarbonate ions (HCO3-) 重碳酸鹽 HCO3- is formed from CO2 within erythrocytes (RBC) 紅血球 which contain carbonic anhydrase (CA) 碳酸酐脫水酶, an enzyme catalyzes the reversible reaction that converts CO2 and water (H2O) to carbonic acid (H2CO3) 碳酸 H2CO3 continues with reversible dissociation of bicarbonate ion (HCO3-) CO2 + H2O CA H2CO3 H+ + HCO3- Law of Mass Action 質量作用定律 an increase in CO2 causes an increase in HCO3- and H+ In addition to being important in the transport and exchange of CO2, this reaction is also important in acid-base balance PCO2, acidity of the blood P491 Carbon dioxide exchange and transport in systemic capillaries and veins The CO2 produced in respiring cells diffuses, based on its partial pressure gradient, first into interstitial fluid, plasma and then into the RBCs Although some CO2 remains dissolved in the blood, and some binds to Hb, most of the CO2 is converted to HCO3and H+ by actions of carbonic anhydrase in the RBCs As HCO3- levels in RBC increases, HCO3- are transported into the plasma in exchange to chloride ions (Cl-) via a transport protein in the RBC plasma membrane H+ is buffered by binding to Hb The couple movement of Cl- into the RBC and HCO3- into the plasma is called chloride shift 氯移轉 Figure 17.11 Carbon dioxide P492-493 exchange and transport in blood. Carbon dioxide exchange and transport in pulmonary capillaries and veins In the lungs, CO2 diffuses down its pressure gradient from blood to alveolar air to be exhaled decreasing the PCO2 in blood As PCO2 in the RBC decreases, HCO3- enters the RBCs coupled with Cl- enters the plasma, and H+ are released from Hb The HCO3 and H+ are then converted by carbonic anhydrase (CA) to CO2 , which diffuses into the alveoli to be exhaled P492-493 Figure 17.11 Carbon dioxide exchange and transport in blood. Effect of oxygen on carbon dioxide transport Increased PO2 in blood decreases the affinity of Hb for CO2, which decreases the ability of the blood to transport CO2 At a given PCO2 (point A), the CO2 content of the blood falls as the PO2 rises (compared points B and C), a phenomenon known as the Haldane effect In respiring tissues, where PO2 is low and PCO2 is high, the Haldane effect promotes the loading of CO2 onto Hb while both the Bohr effect (effect of pH on Hb’s affinity for O2) and the carbamino effect (effect of CO2 levels on Hb’s affinity for O2) work to promote O2 unloading In the lungs, where PO2 is high and PCO2 is low, the Haldane effect promotes unloading of CO2 while the Bohr effect and the carbamino effect promote O2 loading Figure 17.12 Effect of PO2 on carbon dioxide transport. P493 Summary of oxygen and carbon dioxide loading/unloading in tissue & in lungs Figure 17.12 Effects of PO2 and PCO2 on carbon dioxide and oxygen loading and unloading transport. (a) In systemic tissues, as PO2 decreases and PCO2 increases, CO2 loading and O2 unloading occur. (b) In the lungs, as PO2 increases and PCO2 decreases, CO2 unloading and O2 loading occur. P494 V. Central regulation of ventilation The job of the respiratory system is to deliver O2 and remove CO2 from cells at a rate sufficient to keep up with metabolic demands The signals that indicate whether the respiratory system is doing this job adequately are the partial pressures of O2 and CO2 in systemic arterial blood To maintain normal arterial partial pressures, the body must regulate minute alveolar ventilation (VA) 每分鐘肺泡的換氣量 alveolar ventilation depends on the frequency (RR 呼吸速率) and volume of breaths (VT 潮氣容積) P493 Neural control of breathing by motor neurons Breathing 呼吸 is a cycle of inspiration 吸氣 followed by expiration 呼氣 inspiration is an active process that requires contraction of the inspiratory muscles 吸氣肌, and expiration is a passive process in which no muscle contraction is required Because the muscles of respiration are skeletal muscles 骨骼肌, they are stimulated to contract by neural input from somatic motor neuron 體運動神經 the phrenic nerve 橫膈神經 innervates 支配 the diaphragm 橫隔, whereas the internal and external intercostal nerves 內肋間肌及外肋間肌神經 innervates the intercostal muscles 肋間肌 P494 Neural control of breathing by motor neurons Ventilation involves cyclical changes in neural stimulation of respiratory muscles, which cause cyclical changes in lung volume In quiet breathing 平靜呼吸, expiration is a passive process, and thus no neural or muscle activity of the expiratory muscles is present During active ventilation 主動換氣, inspiratory neurons and muscles become more active, and expiratory neurons and muscles become active 橫膈神經 外肋間肌神經 內肋間肌神經 吸氣肌張力 呼氣肌張力 肺容積 Figure 17.14 A comparison of quiet breathing and active ventilation. P494-495 Generation of breathing rhythm in the brainstem Breathing is under both voluntary 隨意 and involuntary 不隨意 control central control of respiration is not fully understood, but research indicates that respiratory control regions 呼吸控制區 are present in the medulla 延腦 and pons 橋腦 of the brainstem 腦幹 Two respiratory control centers are located on each side of the medulla a ventrally located ventral respiratory group (VRG) 腹側呼吸群 and a more dorsally located dorsal respiratory group (DRG) 背側呼吸群 The respiratory center of the pons 橋腦, called the pontine respiratory group 橋腦 呼吸群 (PRG; pneumotaxic center 呼吸 調節中心) may facilitate the transition 轉換 between in inspiration and expiration Copyright © 2008Pearson Education, Inc., publishing as Benjamin Cummings. 中腦 橋腦 延腦 脊髓 橋腦呼吸群 背側呼吸群 腹側呼吸群 Figure 17.15 Brainstem centers of respiratory control. P494-496 Generation of breathing rhythm in the brainstem The central pattern generator (CPG) is a network of neurons that generates a regular, repeating pattern of neural activity called the respiratory rhythm 呼吸節律 The location of the CPG and its mechanism of action are unknown one hypothesis suggests that certain neurons in the CPG have pacemaker 節拍器 activity The figure shows a simplified model for quiet breathing in which the breathing rhythm is produced by the CPG Figure 17.16 Activity of inspiratory neurons. Inspiratory neurons show a ramp 傾斜 increase in the frequency of action potentials during inspiration, followed by a sudden termination of all activity at the end of inspiration and beginning of expiration. P495-496 In this model, respiratory control areas of the medulla are primarily responsible for controlling breathing But breathing is also affected by activity in other brain regions, including the pons, cerebral cortex 大腦皮質, cerebellum 小腦, limbic system 邊緣系統, hypothalamus 下視丘, and medullary cardiovascular regulatory areas 延腦心血管調節區 Several types of sensory input 感學訊息傳入 can alter respiration, presumably through indirect communication with the CPG Figure 17.17 Model of respiratory control during quiet breathing. P496-497 Peripheral Input to Respiratory Centers Several types of sensory input can alter respiration particularly important are signals from central and peripheral chemoreceptors 中樞及週邊的化學 接受器 (chemically sensitive receptor cells) Additional sensory inputs that affect breathing come from a variety of receptors — Pulmonary stretch receptors 肺臟的牽張接受器 in the smooth muscle of pulmonary airways are excited by inflation 膨脹 of the lung and do not appear to play a significant role in regulating breathing in humans — Irritant receptors 刺激接受器 in the lining of the respiratory tract are stimulated by inhaled particulates such as smoke or dust triggers coughing 咳嗽 (irritant receptors in trachea) and sneezing 打噴嚏 (irritant receptors in nose) — Propriceptors 本體接受器 in muscles and joints which detect movement of the body plays a role in stimulating the increase in ventilation that occurs during exercise — Arterial baroreceptors 動脈的感壓接受器 which detect changes in blood pressure — Nociceptors 傷痛接受器 and thermoreceptors 體溫接受器 located throughout the body P496-497 VI. Control of Ventilation by Chemoreceptors Chemoreceptors Changes in chemical concentrations in the blood are detected by chemoreceptors located in major arteries and in the brain, which relay signals to the respiratory control center via afferent neurons 感覺神經 Chemoreceptors detect blood levels of O2 and CO2 relay 轉播 this information to the respiratory control centers Chemoreceptors are classified as either peripheral or central, depending on their location Peripheral chemoreceptors 週邊化學接受器 are located in the carotid bodies 頸動脈體 near the carotid sinus 頸動脈竇 The central chemoreceptors 中樞化學接受器 are located in the medulla oblongata 延腦 P497 Peripheral chemoreceptors Peripheral and central chemoreceptors differ not only in their location but also in their structures and chemical sensitivities 橋腦 延腦 Peripheral chemoreceptors are – specialized chemically sensitive cells that are in direct contact with arterial blood – communicate with afferent neurons projecting to medullary respiratory control regions – respond to changes in PO2, PCO2or pH (which changes when PCO2 changes) 化學接受器的 傳入纖維(舌咽 神經的一部份) 頸動脈體 頸動脈竇 頸動脈竇壁上 的感壓接受器 主動脈弓 主動脈 Figure 17.18 Location of peripheral chemoreceptors in the carotid bodies. Afferents from the chemoreceptors ascend to the medulla, but not directly to the respiratory centers. Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings. P497-498 Peripheral chemoreceptors Decreases in PO2 can directly activate peripheral chemoreceptors, but only when the PO2 drops to less than 60 mmHg O2 is usually not a primary factor in peripheral chemoreceptor activation In fact, changes in H+ concentration are the primary stimulus for peripheral chemoreceptors the main source of H+ is the reaction of CO2 with H2O PCO2 can also activate peripheral chemoreceptors, but most indirectly Figure 17.19 Respiratory control by chemoreceptors. (a) Declining arterial PO2 has little effect on minute ventilation until the PO2 drops to less than 60 mmHg. (b) Increasing arterial PCO2 has large effects on minute ventilation as PCO2 increases above or decreases below normal. At PCO2 greater than 90 mmHg, coma 昏迷 and then death can occur. P497-498 Central chemoreceptors Central chemoreceptors are neurons in the medulla that respond directly to changes in H+ concentration in the CSF 腦脊髓液 surrounding this area H+ cannot cross the blood-brain barrier (BBB) 血腦障壁, but CO2 can CO2 does not effect the central chemoreceptors directly, but instead is converted to H+ and HCO3- by carbonic anhydrase (CA) in the CSF Central chemoreceptors, unlike the peripheral chemoreceptors, are not sensitive to changes in PO2 Respond to [H+] in CSF [H+] depends on [CO2] Not directly responsive to [CO2] Not responsive to [O2] P498-499 Figure 17.20 Activation of central chemoreceptors in the medulla oblongata. Central chemoreceptors respond best to changes in pH in the cerebrospinal fluid (CSF). However, hydrogen ions (H+) cannot cross the blood-brain barrier. Instead, CO2 in the blood diffuses into the CSF, where carbonic anhydrase (CA) catalyzes the conversion of CO2 and H2O to carbonic acid (H2CO3), which dissociates to bicarbonate (HCO3-) and H+. The H+ can then activate the central chemoreceptors. Chemoreceptor reflex A decrease in arterial PO2 to less than 60 mmHg activates the peripheral chemoreceptors but has no effect on the central chemoreceptors An increase in arterial PCO2 activates the peripheral chemoreceptors directly, and both the peripheral and central chemoreceptors after CO2 is converted to H+ and HCO3 A decrease in arterial blood pH (from CO2 or lactic acid produced by cellular metabolism) activates the peripheral chemoreceptors When activate, chemoreceptors stimulate an increase in ventilation, which provides negative feedback to the initial stimulus Figure 17.21 Chemoreceptor reflex: the effects of changes in arterial PO2, PCO2, and pH on ventilation. P499 Chemoreceptor reflex 換氣不足 換氣過度 Figure 17.22 The effects of hypoventilation and hyperventilation on minute ventilation. P500 VII. Local Regulation of Ventilation and Perfusion Ventilation-perfusion ratios In the normal lung, the rate of air flow to the alveoli (ventilation, VA) is matched to the rate of blood flow (perfusion, Q); the dots over the abbreviations indicate that these are rates The relationship of ventilation to perfusion is called the ventilationperfusion ratio and is abbreviated VA/Q in the normal lung, VA/Q is approximately 1 the PO2 and PCO2 of the alveoli are at the normal values of 100 and 40 mmHg Figure 17.23 Ventilationperfusion ratio. P500-501 Ventilation-perfusion ratios When airways are obstructed, for example, VA in certain alveoli decreases, and the blood traveling in the capillaries to those alveoli does not undergo adequate gas exchange VA/Q<1 When pulmonary capillaries are damaged, perfusion is obstructed, causing a decrease in Q VA/Q>1 Figure 17.23 Ventilation-perfusion ratio. (b) If ventilation decreases and perfusion is normal, then VA/Q<1 arterial PO2 , PCO2 . (c) If perfusion decreases and ventilation is normal, then VA/Q>1 arterial PO2 , PCO2 . P500-501 Local control of ventilation and perfusion O2 acts primarily on the pulmonary arterioles 肺臟小動脈 a low PO2 causes a vasoconstriction 血管收縮 ( Q) CO2 acts primarily on the bronchioles 細支氣管 a high PCO2 causes bronchodilation 支氣管擴張 ( VA) P501-502 • If ventilation to certain alveoli decreases: – – – PCO2 and PO2 in blood and air PCO2 in bronchioles bronchodilation PO2 in P. Arterioles vasoconstriction • If perfusion to certain alveoli decreases: – – – PO2 and PCO2 in blood and air PO2 in P. Arterioles vasodilation PCO2 in bronchioles bronchoconstriction Factors Affecting Air Flow & Perfusion of Individual Alveoli Copyright © 2005 Pearson Education, Inc., publishing as Benjamin Cummings. VIII. The Respiratory System in Acid-Base Homeostasis Acid-base disturbances in blood Although the primary function of the respiratory system is to control the O2 and CO2 content of arterial blood, it also plays an important role in regulating the blood’s pH Blood pH is tightly regulated by both respiratory system and kidneys the normal pH range of arterial blood is 7.38~7.42 A change in pH alters the distribution of electrical charge in protein alters protein’s shape and interferes their normal function – acidosis 酸中毒 = blood pH < 7.35 CNS depression – alkalosis 鹼中毒 = blood pH > 7.45 CNS over-excitation P502 The role of the respiratory system in acid-base balance Hb is a buffer緩衝 because it can bind or release H+ HbO2 Hb + O2 Hb + H+ HbH This is important because the tissues are producing CO2, which is quickly converted to HCO3- and H+ some of these H+ can be buffered by Hb Bicarbonate (HCO3-) is another major buffering system in blood if the H+ concentration increases, H+ bind to HCO3- to form CO2 if CO2 levels in blood are allowed to increase, acidosis will result HCO3- + H+ H2CO3 The relationship between CO2 and acidity can be described using Henderson-Hasselbalch equation to maintain normal arterial pH = 7.4 [HCO3-] : [CO2] must be 20:1 CO2 + H2O [HCO3-] pH = 6.1 + log [CO2] P502-504 The role of the respiratory system in acid-base balance The lungs regulate the concentration of CO2, whereas the kidneys regulate the concentration of HCO3- Respiratory acidosis 呼吸性酸中毒 is an increase in the acidity of the blood due to increased CO2, which occurs, for example, during hypoventilation Respiratory alkalosis 呼吸性鹼中毒 is decrease in the acidity of the blood due to decreased CO2, which occurs, for example, during hyperventilation or at high altitudes 高海拔 P504 • Henderson-Hasselbalch equation, which is based on the equilibrium for the dissociation of an acid (HA) into a free hydrogen ion (H+) and a base (A-) H+ + A- HA [H+ ][A-] K= [HA] K[HA] [H+ ]= [A-] [A-] pH = pK + log [HA] Copyright © 2005 Pearson Education, Inc., publishing as Benjamin Cummings.