* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Mechanisms of Alkenes
Asymmetric induction wikipedia , lookup
Discodermolide wikipedia , lookup
Stille reaction wikipedia , lookup
George S. Hammond wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
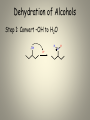
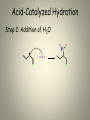
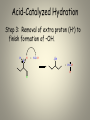
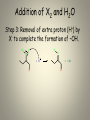
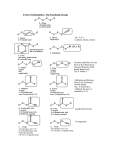
Mechanisms of Alkenes Electrophilic Addition Reactions Understanding the basics… • Mechanisms are the most mind-boggling part of organic chemistry. • Students, generally speaking, have spent their time memorizing their way through science courses. • Mechanisms require a student to actually UNDERSTAND the fundamentals of electron flow… • Everyone knows that electrons are negatively charged. • Everyone knows that electrons are attracted to things with positive charges. • Yet, the understanding of a “mechanism” remains elusive to many students… • Let’s review the basics… • Electron flow is always from the electron-rich to the electron-poor species. • The electron-rich species is a Lewis Base (must have a lone pair) and is called the “nucleophile”. • The electron-poor species is a Lewis Acid (must have empty orbital) and is called the “electrophile”. • SO – electron flow is always from Nucleophile to Electrophile. • Watch the direction of your arrows – from lone pairs to carbocation… – or anion to cation… – or anion to partial positive charge… – or alkene pi bond to cation or partial positive charge… • When working through a mechanism, the goal is NOT to memorize the steps of a mechanism OF A SPECIFIC MOLECULE– when you do that, typically you become too focused on the structures provided in one example. • When that happens, you get confused when the next mechanism problem has a DIFFERENT structure. • What you want to do is break down the steps of the mechanism, into little parts or steps. • These basic little steps can be memorized. • By knowing the steps, you know how the mechanism progresses, regardless of the structure you are given to work with. • SO – break them down… Dehydration of Alcohols Identify this mechanism – Starts with alcohol, ends with alkene… OH H+ + H2O Dehydration of Alcohols Steps Involved: – Convert –OH to H2O – Loss of H2O and Carbocation formation – Removal of H+, resulting in formation of pi bond to complete conversion to alkene Dehydration of Alcohols Step 1: Convert –OH to H2O OH H H+ O H Dehydration of Alcohols Step 2: Loss of H2O (“spontaneous dissociation”) to form carbocation H O H + H-O-H Dehydration of Alcohols Step 3: Removal of H+, resulting in formation of pi bond to complete conversion to alkene + H-O-H H + H-O-H H Acid-Catalyzed Hydration Identify this mechanism – Starts with alkene, ends with alcohol… OH H+, H2O Acid-Catalyzed Hydration Steps Involved: – Reaction of pi bond with H+ (acid cat.) resulting in Carbocation formation – Addition of H2O – Removal of extra proton (H+) to finish formation of –OH. Acid-Catalyzed Hydration Step 1: Reaction of pi bond with H+ (acid cat.) resulting in Carbocation formation H+ H Acid-Catalyzed Hydration Step 2: Addition of H2O H O H + H-O-H H H Acid-Catalyzed Hydration Step 3: Removal of extra proton (H+) to finish formation of –OH. H O H + H-O-H OH + H-O-H H H Addition of H-X Identify this mechanism – Starts with alkene, ends with single halide… X H-X Addition of H-X Steps Involved: – Reaction of pi bond with H+ (of H-X), concurrent separation of X-, and formation of carbocation intermediate. – Attack of X- to finish formation of product. Addition of H-X Step 1: Reaction of pi bond with H+ (of HX), concurrent separation of X-, and formation of carbocation intermediate. H X H + X Addition of H-X Step 2: Attack of X- to finish formation of product. X H X H Addition of X2 Identify this mechanism – Starts with alkene, ends with two halides… X2 X X Addition of X2 Steps Involved: – Attack by pi bond on polarized X-X with Halonium Ion formation – Attack of X- to pop open three-membered ring and finish formation of product. Addition of X2 Step 1: Attack by pi bond on polarized XX with Halonium Ion formation X X + X X Addition of X2 Step 2: Attack of X- to pop open threemembered ring and finish formation of product. X X X X Addition of X2 Of course, you can “attack” the other end of the halonium and open in the other direction… X + X X X Addition of X2 and H2O Identify this mechanism – Starts with alkene, ends with one alcohol and one halide… X2 H2O OH X + HX Addition of X2 and H2O Steps Involved: – Attack by pi bond on polarized X-X with Halonium Ion formation – Attack by H2O to pop open threemembered ring. – Removal of extra proton (H+) by X- to complete the formation of –OH. Addition of X2 and H2O Step 1: Attack by pi bond on polarized XX with Halonium formation X X + X X Addition of X2 and H2O Step 2: Attack by H2O to pop open threemembered ring. H X O H + X + H-O-H X Addition of X2 and H2O Step 3: Removal of extra proton (H+) by X- to complete the formation of –OH. H O H H O + X X + H-X X AND REMEMBER… If you UNDERSTAND the basic steps to these mechanisms, it won’t matter what the double bond in the molecule looks like... Every alkene reacts the same way, every time, regardless of what’s attached… Finally… Track the pieces you need to add or subtract overall… See where you are starting and where you are ending… Don’t memorize a specific molecule but go ahead and memorize the sequence of steps involved… it won’t matter what the alkene looks like if you approach it this way…