* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PDF
Survey
Document related concepts
Transcript
/. Embryol exp. Morph. Vol. 35, 3, pp. 595-606, 1976
Printed in Great Britain
595
A histological study of embryonic
death caused by heterozygosity for the T26H
reciprocal mouse translocation
By P. DE BOER 1 AND P. H. M. D. DE MAAR 1
From the Genetics Department, Agricultural University, Wageningen,
The Netherlands
SUMMARY
Male mice, heterozygous for the T26H reciprocal translocation, were mated to normal
females. A systematic histological study at day 6 and day 8 of pregnancy, covering altogether
303 implantation chambers, yielded the following results: the average number of decidual
reactions per female was 8-91, the average number of morphologically normal embryos at
day 6 was 4-61 and at day 8, 4-25. No significant embryonic loss occurred between day 8
and the end of pregnancy. Most (89-0 %) of the embryonic loss due to genetically unbalanced
(deficiencies combined with duplications) progeny occurs between the moment of the induction of a decidual reaction and day 6, i.e. in ±1-5 days. When the genetically unbalanced
embryos were grouped in three classes according to the size of their deficiencies, the numbers
and types of the aberrant histological pictures found could be reconciled with the expectations of the three classes, based on cytological analysis. According to this, embryos with
the smallest deficiencies take just over 2 days before all have died. Embryos with mediumsized deficiencies take ±1-5 days, and the group with the largest deficiencies take just over
1 day. At day 6, 8-8 % of the implantation chambers had an intact epithelium whereas at
day 8, this had dropped to 1-3 %. This is consistent with the view that erosion of the uterine
epithelium at the site of implantation is an autonomous process, but helped by the phagocytic
action of the trophoblast cells.
INTRODUCTION
Mammalian radiation genetics has mainly used the mouse as its experimental
animal. Radiation-induced reciprocal translocations are characterized by excess
embryonic lethality when a carrier, heterozygous for the translocation (T/+),
is mated to a normal individual. The occurrence of dead implants in such a
situation has even been used as an endpoint for measuring genetic damage (for
instance Searle et al. 1974). No detailed study into the nature of the T/ +
dependent dead implants, observed during the second half of pregnancy, has
hitherto been carried out.
The development of human cytogenetics has yielded numerous cases of
reciprocal translocations (Ford & Clegg, 1969), arousing interest in the timing
and duration of embryonic death accompanying these translocations.
1
Authors' address: Department of Genetics, 53 Generaal Foulkesweg, Wageningen, The
Netherlands.
596
P. DEBOER AND P. H. M. D. DEMAAR
Table 1. Criteria for the developmental age of morphologically normal mouse
embryos of Swiss random-bred stock
Age post-fertilization
(days)
4-5
50
5-5
60
6-5
7-0
7-5
Criteria
Undifferentiated inner cell mass
Early egg-cylinder, with outer proximal endoderm and undifferentiated ectoderm
Appearance of distal endoderm, differentiation into embryonic and
extra-embryonic ectoderm, beginning of ectoplacental cone formation
Formation of the proamniotic cavity, a clearly developed ectoplacental
cone
Formation of the postamniotic fold and the appearance of mesoderm
Formation of the chorion and growth of the primitive streak
Closure of the amnion
The meiotic segregation of the chromosomes involved in a reciprocal translocation yields a definite number of gametic types, all but two characterized by
the presence of a deficiency and a duplication, either separately or combined
(Searle, Ford & Beechey, 1971). These unbalanced gametes are capable of taking
part in fertilization (Ford, 1972) and the resultant unbalanced zygotes will
develop and then die at a particular stage. For the histological study of the
embryological effects of reciprocal translocations in the mouse, we have taken
the translocation T(2; 8)26H which, in heterozygous condition, is known to
produce mainly two types of deficiency /duplication gametes (de Boer, 1976).
The aim of this study was (a) to establish when the genetically unbalanced
embryos die and (b) to see if there is any correlation between the size of the
deficiencies (and duplications) and the time of death.
MATERIALS AND METHODS
Female T(2; 8)26H translocation carriers were of Harwell origin and obtained
through the courtesy of Dr A. G. Searle. The animals were back-crossed to a
Swiss random-bred stock (Cpb:(SE)S) for five generations; T/+ males were
then mated to pure Swiss random-bred females, which were killed on days 6 and
8, yielding embryos of approximately 5 and 7 days old. (The day the vaginal
plug is found is day 1.) Altogether 18 females were killed on day 6 and 16 on
day 8. Care was taken to distribute them as equally as possible over the seven
T/ + males used. In control matings, Swiss random-bred males were mated to
females of the same stock. Autopsy was performed between 9-10 a.m. This
should yield embryos of just over 5 and 7 days old on average, when the dark
period lasts from 6 p.m. to 6 a.m. (Braden, 1957). At autopsy, the uterus and
oviducts were fixed in Bouin's fixative for 3 h. The ovaries were kept for corpora
lutea counts. The uterus was bleached in 70 % ethanol and processed for histo-
Embryonic death due to T26H reciprocal mouse translocation 597
28
X
Fig. 1. Pachytene cross of the T(2; 8)26H reciprocal translocation, which occurs in
heterozygous condition. Chiasmata have been drawn in all four segments of
chromosome pairing. This is the configuration most frequently encountered at
diakinesis-metaphase I (de Boer, 1976). Chromosome 8 segments are dotted.
logical sections at 7 /tm in the usual way. The sections were stained with
Delafield's haematoxylin-eosin. Each deciduoma was sectioned in toto and four
out of every eight sections were prepared for scoring. In our view, the chance
of overlooking an embryo or its remnants is small with this procedure. With the
aid of Snell & Stevens (1966), Rugh (1968) and Theiler (1972), criteria have
been developed for the recognition of normal development with a developmental
stage of 4-5, 5-0, 5-5, 6-0, 6-5, 7-0 and 7-5 days post-fertilization (see Table 1).
Most sections were sagittal to the embryo. Where the embryo was sectioned
transversely, the age of the embryo has been estimated as closely as possible,
with the aid of Snell & Stevens (1966). Implantation chambers were considered
to contain embryonic lethals if embryonic structures were absent or aberrant, or
if the developmental stage was not in concordance with the day of autopsy.
The T26H reciprocal translocation is between chromosomes 2 and 8. The
translocation chromosome 28 is longer than the original chromosome 2 and the
translocation chromosome 82 is shorter than the original chromosome 8 (see
Fig. 1). During prophase of the first meiotic division, homologous chromosome
segments pair (see Fig. 1) and the segregational behaviour of the potential
quadrivalent determines the number of gametic types found. The cytological
analysis of secondary spermatocytes of T26H/ + males gives no definite assessment of the fraction of primary spermatocytes going through translocationcaused numerical non-disjunction, because not all four chromosomes involved
in the translocation can be recognized. The data of de Boer (1976), together with
the pattern of embryonic and foetal death obtained here, suggest that it is low.
The predominant type of segregation is alternate/adjacent-1 (de Boer, 1976),
whereby non-homologous centromeres go to the same pole. This yields two
38
EMB 35
598
P. DE BOER AND P. H. M. D. DE MAAR
Table 2. The garnetic types produced by the T26HJ + translocation during meiosis
An arabic / means the translocated chromosome segment, / the interstitial (from
the centromere to the point of exchange) segment. The percentages of the duplications and deficiencies are averages for a haploid genome, based on a G-banding
analysis (de Boer & van Gijsen, 1974). The percentages of each gametic type assume
an adjacent-2 frequency of 8-5 % (see the Results).
Deficiency
Gametic type
Alternate/adjacent—1
2,8
28, 82
28, 8
2,82
Adjacent—2
2,2
2 ,28
28, 2 s
8 ,8
8 ,82
82, 82
—
—
2t
8,
8
82
8/, 2t
2
2,
/o
Duplication
%
Percentage
—
—
1-2
3-2
2t
3-2
1-2
22-9
22-9
22-9
22-9
4-6
1-4
2-6
6-4
5-2
8-4
2
2<
8 ( , 2,
8
8,:
8,-, 2t
6-4
5-2
8-4
4-6
1-4
2-6
10
21
10
10
21
10
*t
types of balanced gametes (with either two normal or two translocation chromosomes) and two types of unbalanced gametes. Less frequently, homologous
centromeres go to the same pole, i.e. adjacent-2 segregation occurs. This yields
six types of unbalanced gametes. Table 2 summarizes the gametic types thus
produced, the location of the deficiency and duplication and their size according
to measurements in G-banded chromosome preparations (de Boer & van Gijsen,
1974). The gametic frequencies given in Table 2 have been based on cytological
observations on secondary spermatocytes and on relative litter-size data (de
Boer, 1976). The assumption has been made that numerical non-disjunction
caused by translocation is negligible.
The term' small mole' is used for the little blood-filled deciduomata, observed
during the second half of pregnancy (day 14). 'Large mole' refers to a dead
implant, observed at the same day of pregnancy, in which at least the amnion
can be seen macroscopically.
RESULTS
(1) The amount and timing of the embryonic lethality
Table 3 gives mean numbers of corpora lutea, decidual reactions, histologically normal embryos at days 6 and 8 and young born. The difference between
the number of decidual reactions from the two types of mating is not significant
(tn = 0-822). The unbalanced embryos are thus capable of inducing a decidual
reaction, as found by Searle et al. (1971). The difference between the size of
litters sired by T26H/+ males at day 8 and at term is also not significant
ft* = 0-343).
Embryonic death due to T26H reciprocal mouse translocation 599
Table 3. Litter size on days 6 and 8 of gestation {normal embryos only) and at birth
{live and dead), in matings between normal females and either T26H\ + males
or normal males
n is the number of observations on which the mean 3c is based.
Corpora
lutea
9
n
T26H/+X +/ + 34
x +/ + 60
+/+
Decidual
reactions
Day 6
At birth
Day 8
x
n
x
n
x
n
x
n
X
1000
10-37
34
41
8 91
8-55
18
4-61
16
4-25
50
53
408
8-42
Table 4. The distribution of normal and abnormal embryos at day 6 and day 8, in
matings of normal females with T26HJ + males
Histological picture
Day
6
8
Number of uteri
Normal
Abnormal
18
16
83
68
65
87
Total
148
155
Very little further embryonic death, due to translocation-dependent genetic
imbalance or other reasons, was seen after day 8.
A number of females were killed at day 14 to test males for translocation
heterozygosity. In the control, 7 out of 1423 implants had an amnion in which
at superficial inspection the embryo was absent or dead (i.e. a large mole). For
implants from T26H/ + origin, the figures are 6 out of 959. There is no difference
between the two genotypes as to the number of large moles they produce,
confirming that genetic imbalance does not cause embryonic death during the
second half of pregnancy.
Table 4 divides decidual reactions at 6 and 8 days into those yielding a normal
embryo and those containing a dead or retarded embryo {x2 — xl = 4*52,0-025 <
P < 0-05), indicating that a proportion of the total embryonic death occurs
between day 6 and day 8. If the total embryonic death amounts to 8-91 -4-08 =
4-83 decidual reactions (see Table 3) which at the end do not contain a live
embryo, 8-91-4-61/4-83 x 100 = 89-0% occurs between the induction of the
decidual reaction and day 6, an additional 7-5 % occurs between day 6 and day
8 and the remaining 3-5 % between day 8 and the end of pregnancy. Knowing
that 'spontaneous' embryonic lethality (caused by + / + males and + / +
females and observed as a dead implant) is low in the mouse (1-3 % on the basis
of the number of corpora lutea, see Table 3), the 'true' distribution of translocation caused death among the age classes will not deviate much from the
distribution thus given.
600
P. DE BOER AND P. H. M. D. DE MAAR
Table 5. The distribution of the histological/y normal embryos among the age
classes as defined in the Materials and Methods
Developmental age (days)
Autopsy at day 6
Autopsy at day 8
4-5
50
5-5
6-5
70
7-5
24
0
32
0
27
0
0
22
0
32
0
14
Table 6. A schematic representation of histological/y abnormal implantation sites
at day 6 and day 8 of gestation and the percentages of each category as the
fraction of the total number of implantation sites scored
Day 6
Day 8
Category
Category
6.1
6.2.a
6.2.b
6.2.c
6.2.d
6.3
6.4
6.5
Total
Decidual reaction with 13
intact epithelium
Decidual reaction with 7
partially degenerated
epithelium
As above and blood
1
Dissolved epithelium
24
with single
embryonic cells in
the implantation
chamber
As above and blood
3
Embryonic cell clumps
without recognizable
embryonic structures
Abnormal embryonic
structures
Non-classifiable
7
8-8
8.1
4-7
8.2
0-7
16-2
8.3
20
4-7
6
41
3
20
43-2
Decidual reaction with
intact epithelium
Dissolved uterine epithelium, implantation
chamber filled with
blood
As above with single
embryonic cells
8.4.a Embryonic structures
surrounded by blood
8.4.b Abnormally developed
embryos
8.4.c Retarded embryos
8.5
Non-classifiable
Total
2
1-3
60
387
4
2-6
7
4-5
5
3-2
8
1
5-2
0-6
561
Among normal embryos (Table 5), morphological variation with respect to
developmental stage covered three age classes (as defined in the Materials and
Methods). This means a variation in development of between 1-0 and 1-5 days
at each day of gestation.
(2) The histology of abnormal implants
The variation in histological appearance at each stage of pregnancy is listed
in Table 6 and illustrated in Fig. 2. At day 6 of gestation, 13 implantation
chambers showed intact uterine epithelium and no embryonic remnants. An
Embryonic death due to T26H reciprocal mouse translocation 601
[E
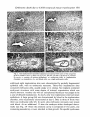
Fig. 2. The implantation chambers depicted correspond with some of the categories
given in Table 6. (A) 6.1, (B) 6.2.a, (C) 6.2.c, (D) 6.4, (E) 8.4.a, (F) 8.4.b. ac, Amniotic cavity; e, erosion of uterine epithelium; ec, embryonic cells; ic, implantation
chamber; m, mesoderm; rbc, red blood cells; sec, single embryonic cells.
additional eight implantation sites were characterized by partially degenerated
epithelial cells, with no embryonic remnants. Thirty-four implantation sites
contained embryonic cells, usually singly or in clumps. Six implants contained
embryonic structures with some degree of internal organization which was
clearly aberrant. Another site with an unimplanted blastocyst was judged to be
a case of delayed implantation. At day 8, only two implantation sites had intact
epithelium. A large group (64) was characterized by blood in the implantation
chamber, only occasionally encountered at day 6. Only in three of those were
there any embryonic cells left. In seven sites embryonic remnants were mixed
with blood. At an additional 13 sites the embryos either developed abnormally (see Fig. 2F where the amniotic cavity is composed of two parts, one
containing mesoderm), or were retarded in their growth. No specific syndromes
602
P. DE BOER AND P. H. M. D. DE MAAR
Table 7. Classification of the gametic types produced by T26HJ + translocation
carriers into classes with correspondingly sized deficiencies
Class 1 contains the largest deficiencies. The frequencies of the classes have been
worked out for 8-5 % adjacent-2 segregation. Implantation classes are used as
defined in Table 6.
Expected
frequency
Percentage
2
3
Deficiency
Duplication
2-6
4-6
1-4
6-4
1-2
28 2 8
8-4
6-4
5-2
4-6
3-2
2-6
2 ,2s
1-4
5-2
2-1]
,8
1-2
3-2
22-9)
540
NJ OO OO 00
1
Gametic
type
NJ OO OO OO
Arbitrary
class
2 82
8-4
c
8 days
6 days
10
10
21 5-1
10
22
^23-9
•25-0
Total
Suggested corresponding
implantation class at
61 (part)
81
8-2 (few)
61 (part)
8-2 (most)
6-2 (most)
6-2 (few)
8-2 (some)
6-3
8-3
6-4 (normal] 8-4
43-2
561
representative of any of the gametic types produced by the T26H/ + male parent
could be traced. The aberrant histological pictures occurred within the same
uteri, as would be expected if (a) the fertilizing capacity of the male gametes is
unrelated to their genetic contents (Ford, 1972) and (b) the embryos with a larger
deficiency die earlier than embryos with a smaller deficiency.
(3) The relation between the spectrum of deviant implantation sites found and the
spectrum of genetically unbalanced zygotes expected
Returning to Table 2, all unbalanced gametes and thus zygotes are characterized by the simultaneous occurrence of deficiencies and duplications, usually of
chromosome segments, but in two cases, originating from adjacent-2 disjunction, of whole chromosomes. From the study of the embryological and foetal
effects of primary monosomies and trisomies (Gropp, Giers & Kolbus, 1974),
it is known that the primary monosomies die considerably earlier than the
primary trisomies. Keeping this in mind, we have classified the gametic types
into three classes, postulating that the embryos with the largest deficiencies die
first. Class 1 contains the types with a deficiency greater than 4-6%, i.e. (2, 2);
(8, 8) and (82, 82). Class 2 contains the types (2, 82) and (28, 28), the latter with a
deficiency of only 2-6% but with a large duplication of 8-4%. Class 3 contains
two types with a small deficiency (2, 28), 1-4% and (28, 8), 1-2%.
Table 7 sets out this classification and gives the expected frequencies with
8-5 % adjacent-2 segregation. This figure has been chosen on the basis of cytological observations in secondary spermatocytes (de Boer, 1976).
Embryonic death due to T26H reciprocal mouse translocation 603
In section 1 of the Results, it has been shown that there is excess embryonic
mortality between days 6 and 8, amounting to 56-1-43-2 = 12-9% (Table 6).
In 12-9 % of the day 8 implants (8.4 of Table 6), embryonic structures were seen.
In 8-8 % of the implants at day 6 there were embryonic cell clumps or abnormal
embryonic structures. Together, this accounts for 21-7 % and makes up most of
class 3 of Table 7. We propose that the embryos of this class start to die before
day 6 and are all abnormal by day 8. Their degeneration would thus take well
over two days. Embryos which give rise to a decidual reaction but do not
contribute to the invasion of the uterine epithelium must die after a shorter time
interval. The blastocyst, but not the morula, is able to evoke a decidual reaction
(McLaren, 1969). Autolysis of the uterine epithelium takes place between 113
and 129 h post coitum (El-Shershaby & Hinchliffe, 1975). If we assume the
blastocyst stage to be reached 3 | days post coitum, these embryos must die in a
period of just over 1 day. At day 6, we found 8-8% of the implantation chambers to be empty with an intact uterine epithelium. We propose that this category
is occupied by the class 1 embryos with perhaps some of the class 2 embryos.
Table 7 suggests that all the embryos of class 2 have died by day 6. The bulk of
this class falls in 6-2 of Table 6 (i.e. about 23-6% of all implants), with few or
no embryonic cells present. Because we also found some more complete
embryonic structures at day 6, the embryos of 6.2 must have been dead for a
couple of hours at the time of autopsy. The data given in Table 6 are consistent
with the assumption that the embryos of class 2 die over a period of 1-5 days.
The total embryonic lethality was 56-1 % at day 8 with probably not much
lethality occurring thereafter. If there is no embryonic lethality between the
blastocyst stage and day 8, other than that associated with translocation
heterozygosity, the expected survival is [%(l-p)]x 100% (Searle et ah 1971),
where p is the fraction of adjacent-2 segregation plus translocation-caused
non-disjunction. WithjP « 0-08 the expected survival is 46% and death 54%.
This is close to the 56-1 % found in this study. Dead implantations occur among
control Swiss matings (see Table 3); although the numbers will be small, it is
therefore not correct to estimate the adjacent-2 frequency from the embryosurvival data in this study.
In section 1 it was reported that the frequency of large moles at day 14 did not
differ between T/+ x + / + and control matings. If numerical non-disjunction
for the translocation-involved chromosomes occurs, a class of zygotes must be
formed mainly trisomic for chromosome 8 or 82 (de Boer, 1976). The latter is
especially likely to be picked up as a large mole (Gropp, Kolbus & Giers, 1975).
The fact that we did not find a surplus of large moles argues against numerical
non-disjunction due to translocation and thus against the production of
trisomic types in T26H/ + males.
604
P. DE BOER AND P. H. M. D. DE MAAR
DISCUSSION
This study is the first in the mouse to locate the period in which embryonic
lethality in translocation heterozygotes occurs, although earlier workers agreed
that it must be shortly after implantation, as judged by the small blood-filled
implantation chambers of rather uniform size, scored at day 10 or later (Snell,
1933; Hertwig, 1940; Carter, Lyon & Phillips, 1955).
The interpretation of the histological sections in terms of genetically unbalanced zygotes, due to heterozygosity for the T(2; 8)26H translocation in the
male parent, has been based on the supposition that embryos with a larger
deficiency die first. Although this seems reasonable to us, there is as yet no
experimental support for this in the mouse or any other mammal, because the
primary monosomies have not yet been studied embryologically. Moreover, the
different primary trisomies show effects during the second half of pregnancy that
are not strictly correlated with their size (Gropp et a/. 1974, 1975). Deficiencies
and duplications of only two chromosomes, 2 and 8, are involved in the present
study. From the primary effects of these chromosomes on development it is
known that monosomic embryos of chromosome 8 must evoke a decidual
reaction and that trisomies for chromosome 8 die relatively early among the
trisomies so far studied. Death occurs about day 11 or 12 (Gropp et a/. 1975).
The fit between the spectrum of morphological descriptions of the implantation
chambers with abnormal or no contents and the spectrum of gametic classes
seems to be good, especially if one realizes that with the number of embryos
employed the standard deviations of the gametic classes and embryonic classes
(Table 6) are rather wide.
Results obtained by Oshimura & Takagi (1975) on embryos karyotyped at
6-5-7-5 days gestation and from matings between T6Ca/+ and normal mice
show that the pattern of death of the unbalanced alternate/adjacent-1 and
numerical non-disjunction types follows the size of their deficiency, large
deficiencies dying first, which is further support for the explanation of our data.
Genetic lethals are more variable at a later stage of development, both as
individuals and as a group. This variation in developmental potential seems to
increase when the genetic imbalance is less severe (Hamerton, 1971; de Boer &
Groen, 1974). On the contrary, when death is brought about by mutant genes
in homozygous condition during the first week of development, the actual
disintegration takes a short period of time (McLaren, 1974).
A human reciprocal translocation with the same embryological consequence
as the T26H mouse reciprocal translocation studied here would lead to embryonic death between days 4 and 17 after fertilization. Abortions in the third week
of pregnancy can be clinically recognized (Boue & Boue, 1974). It is known that
in couples where one parent is the carrier of a reciprocal translocation, there is
only a slight elevation (from ± 15-21-7%) in the level of clinically recognizable
abortions (Ford & Clegg, 1969). This sample of reciprocal translocations was
Embryonic death due to T26H reciprocal mouse translocation 605
biased, however, in that most were ascertained through a defective child,
indicating a non-representative distribution of the translocation breakpoints.
In another, much smaller sample of human reciprocal translocations, not
ascertained through a defective proband, the same tendency was noted, with an
increase in the number of recognizable abortions only at the 5 % probability
level (Jacobs et al. 1970). Thus, the findings in T26H/+ are compatible with
those for human reciprocal translocations, indicating that lethality due to gross
chromosomal imbalance occurs at comparable embryonic stages.
In the present study we found a difference between the fraction of implantation sites with an intact epithelium at day 6 (8-8%) and at day 8 (1-3%).
According to El-Shershaby & Hinchliffe (1975) the invasion of the uterine
epithelium at the implantation site is an autonomous process, although the
trophoblast digests the dead epithelial cells by phagocytosis. This point is
illustrated in our material. The uterine epithelium disappears even when there
is no trophoblast, but the process takes less time when trophoblast is present.
We thank Mr F. A. van der Hoeven for skilled histological assistance. Mr C. V. Beechey
and Dr A. G. Searle commented on the manuscript. Thanks to them as well.
REFERENCES
P. DE (1976). Male meiotic behaviour and male and female litter size in mice with the
T(2; 8)26H and T(l; 13)70H reciprocal translocations. Genet Res. (in the Press).
BOER, P. DE & GIJSEN, M. VAN (1974). The location of the positions of the breakpoints
involved in the T26H and T70H mouse translocations with the aid of Giemsa-banding.
Can. J. Genet. Cytol. 16, 783-788.
BOER, P. DE & GROEN, A. (1974). Fertility and the meiotic behaviour of male T70H tertiary
trisomics of the mouse (Mus musculus). Cytogenet. Cell Genet. 13, 489-510.
BOUE, A. & BOUE, J. (1974). Chromosome abnormalities and abortion. Tn Physiology and
Genetics of Reproduction B (ed. E. M. Coutinho & F. Fuchs). New York and London:
Plenum Press.
BRADEN, A. W. H. (1957). The relationship between the diurnal light cycle and the time of
ovulation in mice. /. exp. Biol. 34, 177-188.
CARTER, T. C , LYON, M. F. & PHILLIPS, R. J. S. (1955). Gene-tagged chromosome translocations in eleven stocks of mice. /. Genet. 53, 154-166.
EL-SHERSHABY, A. M. & HINCHLIFFE, J. R. (1975). Epithelial autolysis during implantation
of the mouse blastocyst: an ultrastructural study. /. Embryol. exp. Morph. 33, 1067-1080.
FORD, C. E. (1972). Gross genome unbalance in mouse spermatozoa: does it influence the
capacity to fertilize. In The Genetics of the Spermatozoon (ed. R. A. Beatty & S. Gluecksohn-Waelsch). Edinburgh, New York: The organizers.
FORD, C. E. & GLEGG, H. M. (1969). Reciprocal translocation. Br. med. Bull. 25, 110-114.
GROPP, A., GIERS, D. & KOLBUS, U. (1974). Trisomy in the fetal backcross progeny of male
and female metacentric heterozygotes of the mouse. Cytogenet. Cell Genet. 13, 511-535.
GROPP, A., KOLBUS, U. & GIERS, D. (1975). Systemic approach to the study of trisomy in the
mouse. IF. Cytogenet. Cell Genet. 14, 42-62.
HAMERTON, J. L. (1971). Human Cytogenetics, vol. II. New York and London: Academic Press.
HERTWIG, P. (1940). Vererbbare Semisterilitat bei Mausen nach Rontgenbestrahlung,
verursacht durch reziproke Chromosomentranslokationen. Z. VererbLehre 79, 1-27.
BOER,
JACOBS, P. E., AITKEN, P., FRACKIEWICZ, A., LAW, P., NEWTON, M. S. & SMITH, P. G.
(1970). The inheritance of translocations in man: data from families ascertained through a
balanced heterozygote. Ann. hum. Genet. 34, 119-131.
606
P. DE BOER AND P. H. M. D. DE MAAR
A. (1969). Stimulus and response during early pregnancy in the mouse. Nature,
Lond. 221, 739-741.
MCLAREN, A. (1974). Embryogenesis. In Physiology and Genetics of Reproduction B (ed.
E. M. Coutinho & F. Fuchs). New York and London: Plenum Press.
OSHIMURA, M. & TAKAGI, N. (1975). Meiotic disjunction in T(14; 15)6Ca heterozygotes and
fate of chromosomally unbalanced gametes in embryonic development. Cytogenet. Cell
Genet. 15, 1-16.
RUGH, R. (1968). The Mouse, its Reproduction and Development. Minneapolis: Burgess
Publ. Comp.
SEARLE, A. G., FORD, C. E. & BEECHEY, C. V. (1971). Meiotic disjunction in mouse translocations and the determination of centromere position. Genet. Res. 18, 215-235.
MCLAREN,
SEARLE, A. G., FORD, C. E., EVANS, E. P., BEECHEY, C. V., BURTENSHAW, M. D. & CLEGG,
H. M. (1974). The induction of translocations in mouse spermatozoa. I. Kinetics of dose
response with acute X-irradiation. Mut. Research 22, 157-174.
SNELL, G. D. (1933). Genetic changes in mice induced by X-rays. Am. Nat. 67, 81.
SNELL, G. D. & STEVENS, L. C. (1966). Early embryology. In Biology of the Laboratory
Mouse. New York: McGraw-Hill Book Company.
THEILER, K. (1972). The House Mouse. Berlin: Springer-Verlag.
{Received 4 December 1975, revised 5 February 1976)