* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Unit 12 Packet
Peptide synthesis wikipedia , lookup
Electrolysis of water wikipedia , lookup
History of electrochemistry wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Citric acid cycle wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Acid throwing wikipedia , lookup
Sulfuric acid wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Nitrocellulose wikipedia , lookup
Thermometric titration wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Biosynthesis wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Biochemistry wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
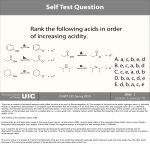
Unit 12: Acids and Bases Name: Acids and Bases 1. Two types of chemicals which have nearly opposite chemical characteristics are acids and bases. They have been known of for some time, mainly because of a collection of contrasting observable properties which made them distinguishable from one another. In the early 1880s, Svante Arrhenius made the first effort to distinguish between acids and bases in terms of their underlying chemistry. The Arrhenius definition of an acid was that an acid is a substance which produces hydrogen ions when it dissolves in water. And for Arrhenius, a base is a substance which produces hydroxide ions when it dissolves in water. (See Masterton and Hurley, pp. 79-80) Using Arrhenius' definition, predict whether the following substances are acids (A), bases (B) or neither (N) based solely upon the chemical formula. Place an A, B or an N in the blank. 2. HCl NaOH Ca(OH)2 CaCl2 HNO3 NH3 NH4 OH HF C5H5N For Arrhenius, acids and bases were electrolytes - substances which dissociated into ions when dissolved in water. Write the balanced chemical equation for the dissociation of the following acids and bases. a. HNO3 (aq) b. NaOH (aq) c. HCN (aq) d. Ca(OH)2 (aq) 3. Review: Rules for naming acids were presented in Chapter 2 (p. 42). The naming rules depend on whether the anion part of the acid contains an oxygen atom. If anion part of the acid does not contain an oxygen atom, then the name takes the form of hydro (anion root name) -ic acid. Thus HF is named as hydrofluoric acid and HCN is named as hydrocyanic acid. If the anion part of the acid contains an oxygen atom, then the name takes the form of either (anion root name) -ic acid or (anion root name) -ous acid. The -ic suffix is used for anions like acetate, phosphate, and chlorate and the -ous suffix is used for anions like sulfite, phosphite, and nitrite. Table 2.2 and Table 2.3 on pp. 39-40 are well worth the look. 4. Like salts (ionic compounds), acids and bases can be strong electrolytes or weak electrolytes. They can completely dissociate in water (strong acids and strong bases) or only partially dissociate in water (weak acids and weak bases). You should know the names and formulas of the following six strong acids and strong bases. (See Masterton and Hurley, p. 81) Formula HCl Name Formula LiOH hydrobromic acid NaOH HI Name potassium hydroxide HNO3 Ca(OH)2 sulfuric acid strontium hydroxide perchloric acid barium hydroxide Page 1 Unit 12: Acids and Bases 5. For the following strong acids and strong bases and their given molarity, write the dissociation reaction and calculate either the hydrogen ion or hydroxide ion concentration. Show your work. a. A 6.0 M HCl(aq) solution: [H+] = b. A 3.0 M NaOH(aq) solution: [OH-] = c. A 1.0 M HBr(aq) solution: [H+] = d. A 1.0 M Ca(OH)2(aq) solution: [OH-] = 6. Recognizing that the Arrhenius definition of acids and bases had several shortcomings, two scientists (Johannes Bronsted and Thomas Lowry) working independently proposed new definitions. The Bronsted-Lowry defintions are: an acid is a proton donor and a base is a proton acceptor. (Masterton and Hurley, pp. 353-354) When dissolved in water, an acid donates a proton (H+ ion) to another substance. When dissolved in water, a base accepts a proton (H+ ion) from another substance. Use these definitions to write the balanced chemical equations for the weak acid and weak base dissociations in water. + HCN(aq) b. HF(aq) c. Dissociation of the weak acid: HNO2 : + H2O(l) a. H2O(l) d. Dissociation of the weak base NH3 (ammonia): e. Dissociation of the weak base C5H5N (pyridine): Page 2 Unit 12: Acids and Bases 7. Name: The Bronsted-Lowry theory of acids and bases perceives every acid-base reaction as being the result of the deprotonation of an acid and the protonation of a base. That is, there is an acid which gives up a proton (H+) and a base which receives the proton (H+). When dissolved in water, it is the water which is doing the accepting (when with an acid) or the donating (when with a base) of the proton. The general equation for the acid-base reaction is: HA acid + B base A- conjugate base + BH+ conjugate acid As noted beneath the equation above, the reaction of an acid with a base changes the acid into a conjugate base and changes the base into a conjugate acid. An acid has one more proton than its conjugate base; a base has one less proton than its conjugate acid. There are always two sets of conjugate acid-base pairs in an acid-base reaction. (Masterton and Hurley, pp. 353, 359-360) Identify the acid (A), base (B), conjugate acid (CA) and conjugate base (CB) in the following acid dissociation, base dissociation and acid-base reactions. Place an A, B, CA or CB in the blanks. 8. a. NH3 _______ + H2O _______ NH4+ _______ + OH_______ b. HCN _______ + H2O _______ CN_______ + H3O+ _______ c. HF _______ + H2O _______ F_______ + H3O+ _______ d. C5H5N _______ + H2O _______ C5H5NH+ _______ + OH_______ e. NH4+ _______ + H2O _______ NH3 _______ + H3O+ _______ f. HCO3_______ + H2O _______ CO32_______ + H3O+ _______ g. HCO3_______ + HF _______ H2CO3 _______ + F_______ f. HSO4_______ + H2O _______ SO42_______ + H3O+ _______ g. HSO4_______ + HCN _______ H2SO4 _______ + CN_______ Some substances are amphiprotic or amphoteric. They can act as either an acid or a base. The most common amphiprotic (amphoteric) substance is water. As seen in the previous question, it acts as an acid when placed with a base. And it acts as a base when placed with an acid. Identify two other substances in question 7 which are amphiprotic (amphoteric). Page 3 Unit 12: Acids and Bases 9. Unlike the strong acids from question #5, a weak acid only partially dissociates. Its dissociation is highly reversible when placed in water, with the dominant species present in solution being the undissociated acid and water. For an acid, the strength of the forward dissociation reaction is described by an equilibrium constant known as an acid dissociation constant (Ka). The same rules which apply to any reversible system apply to the weak acid dissociation reactions. Use the equilibrium constant expression, the Ka value, the dissociation equation and the ICE table to determine ion concentrations for the following solutions of weak acids. (Reference: Chapter 13.4) a. 2.0 M HCN Ka = 6.2 x 10-10 Dissociation Equation: H2O(l) + (aq) (aq) + (aq) Ka Expression: (aq) + (aq) Ka Expression: (aq) + (aq) Ka Expression: I C E Algebra/Solution: b. 1.0 M HC2H3O2 Ka = 1.8 x 10-5 Dissociation Equation: H2O(l) + (aq) I C E Algebra/Solution: c. 0.50 M HNO2 Ka = 4.0 x 10-4 Dissociation Equation: H2O(l) + (aq) I C E Algebra/Solution: Page 4 Unit 12: Acids and Bases 10. Name: Repeat the procedure from question #9 to determine the ion concentrations for these weak bases: a. 0.50 M NH3 Kb = 1.8 x 10-5 Dissociation Equation: H2O(l) + (aq) (aq) + (aq) Kb Expression: (aq) + (aq) Kb Expression: I C E Algebra/Solution: b. 0.020 M CH3 NH2 Kb = 4.38 x 10-4 Dissociation Equation: H2O(l) + (aq) I C E Algebra/Solution: 11. As mentioned earlier, water is amphiprotic (amphoteric) - acts as either an acid or a base. But what's most interesting about water is that it undergoes autoionization as follows H2O(l) + H2O(l) H3O+(aq) + hydronium ion OH-(aq) hydroxide ion The equilibrium constant for this reaction at 25°C is 1.0 x 10-14 . As such, [H3O+] • [OH-] = 1.0 x 10-14 Since the two product ions always establish an equilibrium in any aqueous solution, the product of the hydronium ion concentration and the hydroxide ion concentration is always 1.0 x 10-14 at 25°C. The above mathematical statement is the basis of the so-called pH scale. The pH scale is a logarithmic scale which relates the hydronium ion concentration to a pH value. Higher pH values (greater than 7) correspond to basic solutions. Lower pH values (less than 7) correspond to acidic solutions. A similar scale - the pOH scale - is sometimes used in addition or instead of the pH scale. The following mathematical equations will have to be used for pH, pOH, and [ ] calculations. [H3O+] • [OH-] = 1.0 x 10-14 pH = - log [H3O+] [H3O+] = 10-pH pOH = - log [OH-] [OH-] = 10-pOH pH + pOH = 14.00 Page 5 Unit 12: Acids and Bases 12. Express your understanding of these mathematical relationships by filling in the table. All concentrations are in mol/L or M. Assume a temperature of 25 °C. [H3O+] 0.010 or 1.0 x 10 [OH-] pH pOH Acidic or Basic? -2 0.0010 or 1.0 x 10-3 5.00 11.00 1.0 x 10-7 0.50 or 5.0 x 10-2 6.0 4.0 13. Combine your pH and pOH understanding with your strong acid and strong base understanding (see question #5) to answer the following questions. Assume a temperature of 25 °C. PSAYW a. Calculate the pH of a 2.0 M HCl aqueous solution. b. Calculate the pOH and the pH of a 2.0 M NaOH aqueous solution. c. Calculate the pH of a 0.050 M Ca(OH)2 aqueous solution. d. An aqueous solution of nitric acid is made and found to have a pH of 2.80. Calculate the concentration of the nitric acid in the solution (i.e., before its dissociation). e. Calculate the pH which results when 0.80 g of NaOH are dissolved in 100 mL of water. Page 6 Unit 12: Acids and Bases 14. Name: Historically, acids and bases were distinguished from one another by their observable properties and not by their chemical formulas or their structures. Associated the following observable properties with the acids (A), bases (B) or both (AB). A, B or AB Statement of an Observable Property a. Yields blue on a litmus paper test. b. Conducts an electric current when dissolved in water. c. An aqueous solution will be pink when phenolphthalein indicator is added. d. Tastes sour (though we would never test its taste in lab). e. Tastes bitter (though we would never test its taste in lab). f. Yields red on a litmus paper test. g. Reacts with metals to produce hydrogen gas. h. An aqueous solution will be colorless when phenolphthalein indicator is added. i. May be corrosive. j. Reacts with carbonates to produce carbon dioxide gas. k. Can turn an indicator a different color. Combine your pH and pOH understanding with your weak acid and weak base understanding (see questions #9 and #10) to answer the following questions. Assume a temperature of 25 °C. PSAYW 15. Calculate the pH of a 0.500 M aqueous solutiion of phenolic acid – HOC6H5 (Ka = 1.6 x 10-10). 16. Calculate the pH of a 1.3 M aqueous solution of benzoic acid – HC7H5O2 (Ka = 6.5 x 10-5). Page 7 Unit 12: Acids and Bases 17. Calculate the pH of a 1.5 M aqueous solution of aniline – C6H5NH2 (Kb = 3.8 x 10-10). 18. An aqueous solution of acetic acid – HC2H3O2 (Ka =1.8 x 10-5) - has a pH of 3.4. Determine the acetic acid concentration. 19. Calculate the pH and the %dissociation of a 0.80 M aqueous solution of acetic acid – HC2H3 O2 (Ka = 1.8 x 10-5). 20. Approximately 2.1% of a 1.5 M aqueous solution of a monoprotic acid undergoes dissociation. Calculate the Ka value of the acid. Page 8 Unit 12: Acids and Bases 21. Consider the following groups of acids. Identify the strongest acid (or base) in each group. Identify the weakest acid in each group. By using the word strongest to describe a weak acid, we are refering to the tendency of the acid to dissociate into ions (which will always be weak compared to a strong acid but still be stronger than some other weak acids). a. b. c. 22. Name: HF Ka = 6.6 * 10-4 H3PO4 Ka = 7.1 * 10-3 NH4+ ion Ka = 5.8 * 10-10 Ranking Ranking Ranking HCN Ka = 6.2 * 10-10 H3BO3 Ka = 5.8 * 10-10 HS- ion Ka = 1.3 * 10-13 Ranking Ranking Ranking H2C2O4 Ka = 5.4 * 10-2 CH3COOH Ka = 1.8 * 10-5 CO32- ion Ka = 4.4 * 10-7 Ranking Ranking Ranking As acid-base problems become more and more complex it becomes crucial to be able to read a description of an aqueous solution and to determine what major species are present in the solution. For strong electrolytes (whether salts, acids or bases), it is the dissociated ions which are present in the solution. For weak electrolytes (whether salts, acids or bases), it is primarily the undissociated molecular form of the species which is present. For the following description of aqueous solutions, write the formulas of the major species (present in relatively large concentrations) and of the minor species (present in relatively small concentrations). a. A 2.0 M aqueous solution of NaOH is mixed with a 1.0 M aqueous solution of acetic acid HC2H3 O2 (Ka = 1.8 x 10-5). Major species: Minor species: b. A 2.0 M aqueous solution of HCl is mixed with a 1.0 M aqueous solution of ammonia - NH3 (Kb = 1.8 x 10-5). Major species: Minor species: c. A 0.50 M aqueous solution of NaC2H3 O2 is mixed with a 0.50 M aqueous solution of acetic acid - HC2 H3O2 (Ka = 1.8 x 10-5). Major species: Minor species: d. A 0.20 M aqueous solution of NH4Cl is mixed with a 0.20 M aqueous solution of ammonia NH3 (Kb = 1.8 x 10-5). Major species: Minor species: Page 9 Unit 12: Acids and Bases 23. Volumetric analysis is a technique for determining the amount of a certain substance by doing a titration. In the most common form of a titration, a known volume of a solution of known concentration (the titrant) is added to a known volume of a solution of unknown concentration (the analyte). The addition of the titrant continues until the endpoint is reached. The endpoint is typically signaled by a dramatic change in color (or other observable property) and facilitated by the use of an indicator. If the titration is correctly performed, the endpoint is also the equivalence point or the stoichiometric point - the point in the reaction in which the moles of H+ (or H3O+) ions is equal to the moles of OH- ions. The analysis of a titration problem begins by writing down all the major species present in solution and determining the reaction which occurs. Once the reaction is determined and the balanced chemical equation is written, stoichiometric principles can be used to solve the problem. (Reference: Chapter 4.8 of Masterton and Hurley; introduction on pp. 82-85; Chapter 14.3 with Examples 14.7, 14.8 and 14.9.) a. What volume of 0.250 M NaOH is required to neutralize 50.0 mL of 0.100 M HCl? Major species: Net Ionic Eq'n (balanced): Stoichiometry/Solution: b. A chemistry student finds that 42.1 mL of 0.500 M HCl were required to titrate 20 mL of Ba(OH)2 having an unknown concentration. Determine the concentration of the unknown. Major species: Net Ionic Eq'n (balanced): Stoichiometry/Solution: Page 10 Unit 12: Acids and Bases c. Name: Potassium hydrogen phthalate (KHC8H4 O4, often abbreviated as KHP) has a molar mass of 204.22 g/mol and one acidic proton. In a student titration, 34.67 mL of NaOH with an unknown concentration are used to neutralize 0.2158 g of KHP. Determine the concentration of the NaOH solution. Major species: Net Ionic Eq'n (balanced): Stoichiometry/Solution: d. Baking soda, NaHCO3, is capable of neutralizing stomach acid (mostly HCl). What mass of baking soda is needed to neutralize 198 mL of stomach acid having an HCl concentration of 0.0531 M? Major species: Net Ionic Eq'n (balanced): Stoichiometry/Solution: e. Calculate the mass of aluminum hydroxide required to completely react with 20.0 mL of 0.45M HCl. Major species: Net Ionic Eq'n (balanced): Stoichiometry/Solution: Page 11 Unit 12: Acids and Bases 25. Most acids we’ve studied thus far were monoprotic acids – acids having a single proton to donate. Some acids are polyprotic acids – acids having more than one proton to donate. Examples of polyprotic acids include sulfuric acid (H2SO4), phosphoric acid (H3PO4), carbonic acid (H2CO3), oxylic acid (H2C2 O4), and ascorbic acid (H2C6H6O6). See Table 14.4 on p. 683. Polyprotic acids dissociate in stepwise fashion – one proton at a time. Each dissociation step has its own Ka value; these are usually distinguished from each other by labeling them as Ka1, Ka2, … . Thus, for oxalic acid, the dissociation steps are: H2C2O4 (aq) HC2O4- (aq) H2O(l) + + H2O(l) H3O+ (aq) + HC2O4- (aq) H3O+ (aq) + C2O42- (aq) In general, the first dissociation step occurs more strongly than the second dissociation step. As such, the first step is more significant in terms of affecting the hydrogen ion concentration and the pH. There is however one polyprotic acid which falls in a category of its own – sulfuric acid (H2SO4). Sulfuric acid is a diprotic acid whose first dissociation step occurs completely; that is, the Ka1 value is very large. The second dissociation step is a partial dissociation step and can make an appreciable contribution to the overall hydrogen concentration and pH for any solution with a concentration less than 1.0 M. (Reference: Masterton and Hurley, pp. 366-367) a. Calculate the pH and the concentrations of all species at equilibrium for a 2.0 M solution of carbonic acid (Ka1 = 4.3 x 10-7 and Ka2 = 5.6 x 10-11). Show all your work. Page 12 Unit 12: Acids and Bases b. 26. Name: Calculate the pH and ion concentrations for a 0.250 M solution of sulfuric acid (Ka1 = very large and Ka2 = 1.2 x 10-2). Common Ion Problems: Occassionally a weak acid is dissolved in a solution containing a salt consisting of the conjugate base of the strong acid. That is, the weak acid HA is dissolved in a solution containing NaA or KA or NH4A or … . In such cases, determining the pH of the solution demands that an ICE table is used and the initial concentration of the common anion be included in the ICE table. Solve the following common ion problems using the provided ICE table. Calculate the pH and the percent dissociation of a solution containing 0.50 M HF (Ka = 7.2 x 10-4) and 1.5 M NaF. a. Dissociation Equation: H2O(l) + (aq) (aq) I C E Algebra/Solution: Page 13 + (aq) Ka Expression: Unit 12: Acids and Bases b. Calculate the pH and the percent dissociation of a solution containing 0.10 M HC2H3 O2 (Ka = 1.8 x 10-5) and 0.50 M NaC2H3O2. Dissociation Equation: H2O(l) + (aq) (aq) + (aq) Ka Expression: I C E Algebra/Solution: 27. Question #26 involved analyzing a solution containing a weak acid and the salt of its conjugate base. The same procedure can be used to analyze a weak base and the salt of its conjugate acid. Use the ICE table below to solve the following common ion problem. Calculate the pOH, pH and the percent dissociation of a solution containing 0.25 M NH3 (Kb = 1.8 x 10-5) and 0.10 M NH4Cl. Dissociation Equation: H2O(l) + (aq) (aq) I C E Algebra/Solution: Page 14 + (aq) Kb Expression: Unit 12: Acids and Bases 28. Name: Perhaps the most important application of acid-base chemistry involving common ions in solution is buffering. Buffers are solutions which contain a weak acid and a salt consisting of its conjugate base (or a weak base and a salt consisting of its conjugate acid). By carefully selecting the right collection of a weak acid the conjugate base, a solution can be made which offers a high resistance to a pH change. Even when an acid or base is added to a buffer solution, the solution is very resistant to changes in its pH. Suppose that solutions are made by mixing the following sets of compounds. In which case will the solution act as a buffer? Solution Compos’n 29. Buffer? Solution Compos’n Buffer? HNO2 / NaNO2 Yes No NH3 / NH4Cl Yes No HNO3 / NaNO3 Yes No H2CO3 / NaHCO3 Yes No H2S / NH4 HS Yes No H2SO4 / NaHSO4 Yes No Ca(OH)2 / HCa(OH)2 Yes No NaCl / CaCl2 Yes No The pH of a buffered solution can be determined in the same manner as the pH of a common ion solution is determined – through the use of an ICE table and the equilibrium constant expression. Use an ICE table to solve the following problems. (Reference: Masterton and Hurley, Chapter 14.1) a. Calculate the pH of a buffered solution containing 0.10 M H2 CO3 (Ka = 4.4 x 10-7) and 0.25 M NaHCO3. Dissociation Equation: H2O(l) + (aq) (aq) + (aq) Ka Expression: I C E Algebra/Solution: b. Calculate the pH of a buffered solution containing 0.40 M NH3 (Kb = 1.8 x 10-5) and 0.25 M NH4Cl. Dissociation Equation: H2O(l) + (aq) (aq) I C E Algebra/Solution: Page 15 + (aq) Kb Expression: Unit 12: Acids and Bases 30. When a base is added to a weak acid/conjugate base buffer solution, the solution resists the change in pH because the weak acid neutralizes the base; the weak acid-conjugate base equilibrium shifts in order to replenish the reacted acid and thus maintain roughly the original acid content in the solution. This is simply an application of LeChatelier’s principle. The mathematical analysis of such a process involves two steps: 1) the stoichiometry step in which the amount of weak acid needed to neutralize the base is determined; 2) the equilibrium step in which the LeChatelier shift of the weak acid-conjugate base equilibrium is analyzed. (Reference: Masterton and Hurley, pp. 388- 390) Consider the buffer solution from question #29a: 0.50 M H2 CO3 (Kb = 4.4 x 10-7) and 0.75 M NaHCO3. Determine the new pH value which would result if 0.05 moles of NaOH is added to 1 liter of this solution. As a contrast, determine the pH if 0.05 moles of NaOH is added to 1.0 L of pure water. Page 16 Unit 12: Acids and Bases 31. Name: The concept of a conjugate acid and a conjugate base was discussed in question #7. Now consider the following truths about the conjugates of acids and bases. • • • • The conjugate base of a weak acid tends to form basic solutions in water. The conjugate acid of a weak base tends to form acidic solutions in water. The conjugate base of a strong acid has neither acidic or basic properties. The conjugate acid of a strong base has neither acidic or basic properties. Identify the following ions as being either acidic, basic or neutral. 32. Species Circle Species Circle NO2 - acidic basic neutral NO3 - acidic basic neutral C2H3O2 - acidic basic neutral NH4 + acidic basic neutral HS - acidic basic neutral OH - acidic basic neutral Cl - acidic basic neutral F- acidic basic neutral Salts are ionic compounds consisting of a cation (+ ion) and an anion (- ion). Salts can actually produce acidic or basic solutions when dissolved in water, depending on whether the cation or anion is the conjugate base or acid of a weak acid or weak base. Since an ion which is the conjugate acid of a weak base is a strong acid, a salt containing this ion will produce an acidic solution. Similarly, since an ion which is the conjugate base of a weak acid is a strong base, a salt containing this ion will produce an basic solution. Complete the following statements, thus outlining the logic which explains whether a salt would form an acidic, basic or neutral solution. (Reference: Masterton and Hurley, pp. 372-373) a. The cation NH4+ is the conjugate _______________ of the ______________ _____________ NH3. For this reason, the salt NH4Cl would dissolve in water to form a(n) ________________ (acidic, basic, neutral) solution. b. The anion F- is the conjugate _______________ of the ______________ _____________ HF. For this reason, the salt NH4Cl would dissolve in water to form a(n) ________________ (acidic, basic, neutral) solution. c. The anion NO3- is the conjugate _______________ of the ______________ _____________ HNO3. For this reason, the salt NaNO3 would dissolve in water to form a(n) ________________ (acidic, basic, neutral) solution. d. The cation Ca2+ is the conjugate _______________ of the ______________ _____________ Ca(OH)2. For this reason, the salt CaCl2 would dissolve in water to form a(n) ________________ (acidic, basic, neutral) solution. 33. Categorize the following salts as forming acidic, basic or neutral solutions when dissolved in water. (Reference: Masterton and Hurley, pp. 372-373) Species NaCN Circle acidic basic neutral Species KNO3 Circle acidic basic neutral NaCl acidic basic neutral NH4NO3 acidic basic neutral NaC2H3O2 acidic basic neutral NH4C2H3 O2 acidic basic neutral Page 17 Unit 12: Acids and Bases 34. The pH of a salt solution can be determined if the Ka value of its weak acid conjugate is known. The underlying principle which allows for such a calculation is that the Kb value of the conjugate base of a weak acid is Kw / Ka (where Kw is 1.0 x 10-14). As an example, if the Ka for HCN is 6.2 x 10-10, then the Kb of CN- (the conjugate base of HCN) is (1.0 x 10-14 / 6.2 x 10-10) = 1.6 x 10-5. Use this information to solve the following problems. a. Calculate the pH of a 0.50 M NaCN solution. The Ka HCN is 6.2 x 10-10. Dissociation Equation: H2O(l) + (aq) (aq) + (aq) Kb Expression: I C E Algebra/Solution: b. Calculate the pH of a 2.0 M NaC2H3O2 solution. The Ka HC2H3O2 is 1.8 x 10-5. Dissociation Equation: H2O(l) + (aq) (aq) I C E Algebra/Solution: Page 18 + (aq) Kb Expression: Unit 12: Acids and Bases 35. Name: The procedure used in question #34 for the conjugate of a weak acid can also be used for the conjugate of a weak base. Calculate the pH of a 0.25 M solution of ammonium nitrate. The Kb of ammonia (NH3) is 1.8 x 10-5. Dissociation Equation: H2O(l) + (aq) (aq) + (aq) Ka Expression: I C E Algebra/Solution: 36. As seen in question #35, a salt containing a cation which is the conjugate acid of a weak base produces an acidic solution. A second type of salt which produces an acidic solution is one which contains a highly charged metal ion such as Al3+ or Fe3+ or Cr3+. When present in water, the cation becomes quickly hydrated and its hydrated form is slightly acidic. Determine the pH of a 2.5 M iron(III) chloride solution. The Ka value of Fe(H2O)63+ is 6.0 x 10-3. Dissociation Equation: H2O(l) + (aq) (aq) I C E Algebra/Solution: Page 19 + (aq) Ka Expression: Unit 12: Acids and Bases 37. Strong Acid-Strong Base Titration: Consider the titration of 20.0 mL of 0.500 M HNO3 with 0.250 M NaOH. Determine the pH value of the resulting solution after the addition of the following amounts of base to the acid. (Reference: Masterton and Hurley, pp. 394-395; Example 14.7) Volume NaOH (mL) pH Please show your work. 0.00 10.0 20.0 30.0 35.0 38.0 40.0 42.0 45.0 50.0 60.0 Construct a plot of pH vs. volume of acid used. Be sure to label axes values. Identify the endpoint with a large • on the diagram. What is the pH value at the endpoint of a strong acid-strong base titration? Use Figure 14.4 and Table 14.2 to identify a suitable indicator for the detection of this endpoint. Page 20 Unit 12: Acids and Bases 38. Name: Weak Acid-Strong Base Titration: Consider the titration of 20.0 mL of 0.500 M HC2H3O2 (Ka = 1.8 x 10-5) with 0.250 M NaOH. Determine the pH value of the resulting solution after the addition of the following amounts of base to the acid. (Reference: Masterton and Hurley, pp. 396-397; Example 14.8) Volume NaOH (mL) pH Please show your work. 0.00 10.0 20.0 30.0 35.0 38.0 40.0 42.0 45.0 50.0 60.0 Construct a plot of pH vs. volume of acid used. Be sure to label axes values. Identify the endpoint with a large • on the diagram. What is the pH value at the endpoint of a weak acid-strong base titration? Use Figure 14.4 and Table 14.2 to identify a suitable indicator for the detection of this endpoint. Page 21 Unit 12: Acids and Bases 39. Weak Base-Strong Acid Titration: Consider the titration of 20.0 mL of 0.500 M NH3 (Kb = 1.8 x 10-5) with 0.250 M HCl. Determine the pH value of the resulting solution after the addition of the following amounts of acid to the base. (Reference: Masterton and Hurley, pp. 397-398) Volume HCl (mL) pH Please show your work. 0.00 10.0 20.0 30.0 35.0 38.0 40.0 42.0 45.0 50.0 60.0 Construct a plot of pH vs. volume of acid used. Be sure to label axes values. Identify the endpoint with a large • on the diagram. What is the pH value at the endpoint of a weak base-strong acid titration? Use Figure 14.4 and Table 14.2 to identify a suitable indicator for the detection of this endpoint. Page 22 Unit 12: Acids and Bases Name: 26. Hydrolysis of Salts 27. Structure and Acidity and Acidic properties of Oxides (Chapter 14.9-10) 29. Introduce buffer problems and repeat the common ion; do some conceptual Qs on preparing buffers 30. pH curves (might save this for last - Chapter 15:4-5) Page 23 Unit 12: Acids and Bases References: 1. Wikipedia on Definitions of Acids and Bases: http://en.wikipedia.org/wiki/Acid-base_reaction_theories 2. Identifying conjugate acid-base pairs (practice with answers): http://chemistryandphysics.astate.edu/draganjac/AcidBasePairs.html 3. Acid-Base Titration: http://preparatorychemistry.com/Bishop_Titration.htm Page 24