* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Acid and Bases 2
Survey
Document related concepts
Transcript
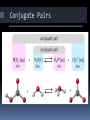
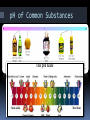
Chapter 19 Notes: Part II Acid/Base Theories There are three ways to define acids and bases. This reflects the fact that science is always revising itself. 1) Arrhenius Acids/Bases An acid is a chemical that gives off hydrogen ions in solution. A base is a chemical that gives off hydroxide ions in solution. Example: HCl + NaOH NaCl + H2O **BASICALLY…acids start with H+ and bases end with OH-!!! Arrhenius Acids/Bases There are three ways to describe an acid: I. Monoprotic-gives off one H+ in sol’n II. Diprotic-gives off two H+ III. Triprotic-gives off three H+ Bronsted-Lowry Acids/Bases The Arrhenius definition is a good one, but does not encompass everything that shows acidic/basic qualities. To account for this, Johannes Bronsted and Thomas Lowry proposed a new idea. 2) Bronsted-Lowry Acids/Bases An acid is a hydrogen-ion (proton) donor. A base is a hydrogen-ion (proton) acceptor. Conjugate Acids/Bases A conjugate acid is the particle that is formed when a base gains a hydrogen ion. A conjugate base is the particle that is formed when an acid donates a hydrogen ion. Conjugate Acids/Bases A conjugate acid/base pair consist of two substances related by the loss of a single hydrogen ion. Conjugate Pairs NH3 + H2O NH4 + + OH proton donator Which is the initial acid? H2O proton acceptor Which is the initial base? formed after base gains H+ What is the conjugate acid? formed after acid donates H+ NH3 NH4+ What is the conjugate base? OH- HCl + H2O H3 + O + Which is the initial acid? Which is the initial base? What is the conjugate acid? What is the conjugate base? Cl HCl H2O H3O+ Cl- Amphoteric Substances Note that in one of the previous examples H2O was acidic and basic in the other. A substance that can act as an acid or a base is called amphoteric. 3) Lewis Acids/Bases A Lewis acid is a substance that can accept a pair of electrons to form a covalent bond. A Lewis base is a substance that can donate a pair of electrons to a covalent bond. **MUST draw Lewis dot structures to determine whether a compound will gain or lose e-!!! 7 5 1 Ex… BF3 + NH3 x x xx xx Fx x xB F xx x x xFx xx x x x x ACID (not happy) accept e- pair xH HN x H x BASE (happy) donate e- pair Hx N x H x H 3 F3BNH3 x x xx xx Fx x xB F xx x x xFx xx x x x x pH scale The pH scale is a way of expressing the strength of acids and bases. Instead of using very small numbers based on the Molarity of the H+ (or OH-) ion , We use the NEGATIVE power of 10 on the Molarity of the H+ (or OH-) ion. pH = - log [H+] Under 7 = acid 7 = neutral Over 7 = base The Inverse Relationship between the pH and pOH Scales For any neutral solution, pH + pOH = 14.00 (at 25°C) pH of Common Substances pH [H+] [OH-] pOH