* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Physics 228 Today: April 22, 2012 Ch. 43 Nuclear
Survey
Document related concepts
Nuclear and radiation accidents and incidents wikipedia , lookup
Nuclear magnetic resonance spectroscopy wikipedia , lookup
Nuclear magnetic resonance wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Nuclear fission wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Two-dimensional nuclear magnetic resonance spectroscopy wikipedia , lookup
Nuclear fusion wikipedia , lookup
Valley of stability wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Transcript
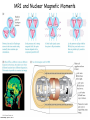
Physics 228 Today: April 22, 2012 Ch. 43 Nuclear Physics Website: Sakai 01:750:228 or www.physics.rutgers.edu/ugrad/228 Wednesday, April 24, 2013 Nuclear Sizes Nuclei occupy the center of the atom. We can view them as being more or less spherical, with a typical radius of a few times 10-15 m - a few fm (pronounced femtometer or "Fermis"). Studies of nuclei have revealed that the protons and neutrons, are strongly attracted to each other, with the result that they are packed densely into a nucleus. It is a semi-reasonable approximation for nuclei to consider them to be spherical, and made up of dense hard-packed spheres, protons and neutrons. Packing A spheres into a nucleus gives a volume proportional to A, and a radius proportional to A1/3. It has been found that the radius is about r = R0A1/3 with R0 = 1.2 fm. Nuclear matter is the densest matter known. For normal nuclei, ρ ≈ 0.14 nucleons/fm3 ≈ 2.3x1017 kg/m3, vs 103 kg/m3 for water and about 104 kg/m3 for the densest atoms. Neutron stars are believed to be about 5 times denser than normal nuclei, based on theoretical extrapolations. (Note neutron stars exist because they are bound by gravity, not the nuclear force.) Wednesday, April 24, 2013 Nuclear Masses It is a good starting approximation that the mass of a nucleus is simply proportional to the number of nucleons in the nucleus, A. But the nucleons are bound into the nucleus, so the mass of the nucleus is less than the sum of the masses of the constituent nucleons. The mass of a nucleus is roughly proportional to the number of nucleons times 931.5 MeV = 1.66x10-27 kg = 1 u, an atomic mass unit. You can see from this that nucleons are bound into a nucleus by ≈ 939 - 931 = 8 MeV, about 1% of their mass. The a.m.u. is defined so that the mass of the 12C atom is exactly 12. Note we have the same thing for electrons in atoms, the mass of the atom is less than the mass of its constituents, but there the effect is about 10 eV / 511,000 eV, or about 2x10-3%, if we use the electron mass, or much less using the atomic mass. Wednesday, April 24, 2013 Binding Energy In determining nuclear masses, we usually deal with atoms, so tabulated masses are usually for atoms. The atomic electron masses and binding energy make a small difference, but usually if we ignore the inclusion of electrons it is not an issue, since the number of nucleons and electrons is constant for all processes we will consider here. The total nuclear binding energy is the total energy needed to separate a nucleus into its constituents, Z free hydrogen atoms plus N free neutrons: EB = (ZmH + Nmn - AM)c2. One can also refer to the binding energy of the most loosely bound proton or neutron. If all the masses are known in a.m.u., the binding energy calculations shown is straightforward, with the binding energy typically given in MeV. This uses mp = 1.007825 u, mn = 1.008665 u, md = 2.014102 u, etc. The deuteron binding energy is 1.007825 u + 1.008665 u - 2.014102 u = 0.002388 u = 2.224 MeV. Wednesday, April 24, 2013 Mass Excess? Since the nuclear mass is nearly A u, and since the number of nucleons is conserved, it is common for practicing nuclear physicists to use the concept of mass excess. The mass excess Δ is the difference between the mass and the approximation that the mass is A u. To have numbers of order 1, units of MeV are used. The proton mass excess is 7.289 MeV. The neutron mass excess is 8.071 MeV. The deuteron mass excess is 13.136 MeV. Thus, the binding energy for the deuteron is 7.289+8.071-13.136 = 2.224 MeV. Since the number of nucleons is constant, the two average nucleon masses would be added and subtracted, and thus cancel out, if we included them. Note also to get the deuteron mass using amu, we needed to use 7-digit long numbers. Here we needed 5 digits to get the same precision answer, so it is more convenient. Wednesday, April 24, 2013 Binding Energy / Nucleon The total binding energy divided by the number of nucleons is the binding energy per nucleon. It is maximum for medium mass nuclei. Thus, if two light nuclei fuse into a heavier nucleus, energy is released. Or if a heavy nucleus splits into two light ones, energy is released. This pattern provides the underlying reason why the sun generates light, and why nuclear reactors and weapons work. Wednesday, April 24, 2013 The jagged pattern is due to QM shell effects. Saturation The pattern of the binding energy per nucleon being about constant is an indication of the saturation of the nuclear force. The force is short ranged, about 1 - 2 fm, so each nucleon largely interacts with its nearest neighbors rather than with the nucleus as a whole. As a result, nucleons in large nuclei all have about the same number of neighbors and the binding energy per nucleon is about constant. Wednesday, April 24, 2013 The jagged pattern is due to QM shell effects. The Nuclear Force The textbook, on the nuclear force: "physicists have yet to determine its dependence on the separation r." This is somewhat misleading. In old fashioned mid-1900s quantum mechanics the force between nucleons arises from nucleons exchanging pions. This has a definite spatial dependence: UYukawa = -C e-mr / r. The exponential converts a Coulomb-type potential into a short range "screened" Coulomb potential. The mass of the pion appears as the factor of m in the exponential. This idea arises nicely from Heisenberg's uncertainty principle. A "virtual" photon exchanged in the Coulomb interaction can have m = ΔE ≈ 0, so Δt can be ≈ ∞, you can have a long range force. The pion mass is 140 MeV, so a "virtual" pion can live only a short time and travel a short range. Crudely... Δx = cΔt = ħc/2ΔE = 200 MeV.fm / 300 MeV ≈ 1 fm. Wednesday, April 24, 2013 The Nuclear Force Now it is also understood that subatomic forces cannot be expressed only as functions of r. They have terms that depend on r (central force), terms that depend on momentum (derivatives of positions, if you think back to the Schrödinger Eq.) (nonlocal force), and terms that depend on the alignment of the spins of the two nucleons (tensor force), and terms that depend on the alignment of the spin and orbital angular momentum of the two nucleons (spin-orbit force). You might recall things of this sort from our discussions of the EM force and atomic structure. Wednesday, April 24, 2013 iClicker By what factor is the radius of the nucleus 160Dy bigger than that of 20Ne? a) <1 - because of the strength of the nuclear force, nuclei get smaller as you add more nucleons b) 1 - all nuclei are about the same size c) 2 d) 4 e) 8 Wednesday, April 24, 2013 Nucleon Magnetic Moments Protons, neutrons, and nuclei also have magnetic moments. Protons and neutrons are spin-1/2, the same as the electron. For the proton, similar to the electron, we would expect if it is a point-like particle for its magnetic moment to be one nuclear magneton, defined as μn = eħ/2mp = 3.152x10-8 eV/T. For the neutron, since it is charge 0, we would expect it to have no magnetic moment. But actually protons and neutrons have a complex internal structure, as do nuclei and atoms, so their magnetic moments are not so simply related to their spins. For the proton, μ = 2.793 μn, while for the neutron, μ = -1.913 μn. The "-" sign for the neutron indicates that its magnetic moment is directed opposite to its spin, unlike the proton. Wednesday, April 24, 2013 Nuclear Magnetic Moments For the proton, μ = 2.793 μn, while for the neutron, μ = -1.913 μn. The "-" sign for the neutron indicates that its magnetic moment is directed opposite to its spin, unlike the proton and electron. Nuclear magnetic moments arise from the proton and neutron moments and their orbital motions. As with the case of atomic electrons, most nucleons form pairs whose total angular momentum, and total magnetic moment, add to 0. As a result, nuclear magnetic moments often result from only a few nucleons, and are typically of size a few nuclear magnetons. In addition, particularly for heavier nuclei, the "entire" nucleus can rotate leading to orbital angular momenta, like for molecules. Wednesday, April 24, 2013 MRI and Nuclear Magnetic Moments Wednesday, April 24, 2013 The Liquid Drop Model An old idea is that nuclei act like quantum liquids (George Gamow, 1928). This leads to the "semi-empirical mass formula": 2 Z(Z − 1) (A − 2Z) 2/3 −4/3 EB = C1 A − C2 A − C3 − C ± C A 4 5 A A1/3 C1: 16 MeV - the binding of a nucleon to a complete set of nearest neighbors C2: 18 MeV - nucleons on the surface do not have neighbors outside to bind to C3: 0.7 MeV - the Coulomb potential energy of protons C4: 24 MeV - the symmetry energy... because there are different states for protons and for neutrons, nuclei with N ≈ Z are more bound (neglecting Coulomb) C5: 39 MeV - protons bound in pairs and neutrons bound in pairs are more bound in nuclei (the two of a pair are in the same quantum state); this term is positive for even numbers of both, negative for odd numbers of both, and 0 for one type of nucleon odd, the other even Wednesday, April 24, 2013 Odd and Even Nuclei 2 Z(Z − 1) (A − 2Z) 2/3 −4/3 EB = C1 A − C2 A − C3 − C ± C A 4 5 A A1/3 Nuclear pairing is such an important feature that most nuclei in nature have even numbers of protons and neutrons. A significant fraction are odd-even, with an even number of one of the nucleon types, and an odd number of the other. But there are only a few stable odd-odd nuclei. These are 2H, 6Li, 10B, and 14N, all light nuclei which we can better understand from ideas of finite quantum mechanical potential wells than from the idea of a liquid drop. Wednesday, April 24, 2013 Vibrations and Rotations Thinking of nuclei as liquid drops, you can imagine classically how the liquid can have various vibrations and rotations. There are in fact quantum vibrational, "(n+1/2)ħω", and rotational, l(l+1)ħ2/2I spectra, particularly for heavier nuclei. Wednesday, April 24, 2013 The Shell Model The main "central" nuclear potential is shaped something like a finite square well. Because of the curvature it is often thought of as a finite harmonic oscillator well. This leads to the idea of a set of nuclear states just like the states of a finite well. For the atom, we found that for particular numbers of electrons, major atomic shells (K, L, M, ...) were filled and the atoms were particularly stable and non-reacting. For nuclei, a similar phenomena is observed. This leads to the "shell" model getting the spacing about right requires using mainly the central force and a spinorbit force, that lowers the energy of orbits where S || L, not anti-parallel. Wednesday, April 24, 2013 Magic Numbers For atoms, the "magic numbers" were Z=2, 10, 18, 36 corresponding to filling 1s, 2s+2p, 3s+3p, 4s+4p+3d, ... shells. For nuclei, the typical naming of orbits is different, the order in which they fill is different, and the doubly "magic nuclei" are 4He with 1s1/2 shell filled for p+n, 16O with 1s1/2 + 1p3/2 + 1p1/2 shell filled for p+n, 40Ca with ... + 1d5/2 + 2s1/2 + 1d3/2 orbits filled for p+n, ... 48Ca with ... + 1f7/2 filled with n, ... These nuclei have higher binding energies than their neighbors, and also the excited states are further away from the ground states than non-magic nuclei. Wednesday, April 24, 2013 iClicker Which of the following nuclei likely has the largest binding energy per nucleon? a) 15 b) 14 N 7 c) 12 d) 13 e) 16 7N 6C 6C 8O Wednesday, April 24, 2013