* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download FUNCTIONAL AMINO ACIDS AND INTESTINAL IMMUNE
Survey
Document related concepts
Transcript
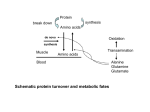
VI Congresso Latino-Americano de Nutrição Animal - SALA SUÍNOS 23 a 26 de Setembro de 2014 – Estância de São Pedro, SP - Brasil Realização: Colégio Brasileiro de Nutrição Animal - CBNA FUNCTIONAL AMINO ACIDS AND INTESTINAL IMMUNE FUNCTION IN NEONATES MALCOLM WATFORD, D.Phil. Department of Nutritional Sciences School of Environmental and Biological Sciences Rutgers University - New Brunswick NJ 08904 - USA Malcolm Watford, D.Phil. Department of Nutritional Sciences 96 Lipman Drive SEBS Rutgers University New Brunswick NJ 08904 USA 848-932-7418 [email protected] Abstract The gastrointestinal tract and the gut associated lymphoid system are critical to maintain both adequate nutrient absorption and defense against pathogenic microbes. In the piglet however, these systems are relatively immature at birth and develop over first six weeks of post-natal life. The introduction of early abrupt weaning places an enormous stress on piglets that is reflected in decreased food intake, excessive diarrhea and loss of weight. Glutamine (and glutamate) and arginine are required by both intestinal and immune cells for many vital functions. Supplementation of these amino acids has been shown to be beneficial to early weaned piglets, in that they reduce diarrhea, maintain both intestinal morphology and function, and enhance immune function, particularly under conditions of oxidative stress or bacterial infection. Furthermore, provision of these amino acids prior to weaning also has beneficial outcomes, indicating that they are limiting both in porcine milk and in the ability of the piglet to synthesize them endogenously. A limited number of studies have shown that supplemental glutamine to the sow during lactation results in higher glutamine concentrations in the milk which is predicted to be beneficial to the piglets. In addition, such supplemental glutamine appears to prevent the loss of lean body mass that normally occurs in sows during lactation. Thus at least, glutamine and VI Congresso Latino-Americano de Nutrição Animal - SALA SUÍNOS 23 a 26 de Setembro de 2014 – Estância de São Pedro, SP - Brasil Realização: Colégio Brasileiro de Nutrição Animal - CBNA arginine should be reclassified a functional amino acids, ones that offer benefits when included in the diet at specific times in the life cycle. Introduction Traditionally dietary amino acids have been described as essential (non-dispensable) or non-essential (dispensable), referring to the capacity of the body to synthesize the specific amino acid in sufficient amounts to support maximal growth or maintain nitrogen balance [1]. This definition was, in part, due to the technical limitations at the time the individual amino acids were first isolated and used in dietary studies. As we have become more aware of important roles of amino acids other than simply substrates for protein synthesis, it has become apparent that providing an amino acid at levels that support growth and nitrogen balance falls short of providing an amino acid for other functions. Thus modern considerations of dietary amino acid requirements are increasingly being assessed in terms of their non-protein roles and some non-essential amino acids are being reclassified as functional amino acids [2,3]. Two important, non-protein, roles of such functional amino acids are to maintain intestinal integrity (barrier function) and to support the immune system. These functions are linked in that the epithelial cells of the gut are considered to be part of the innate immune system and thus comprise part of the gutassociated lymphoid tissue (GALT) [4,5]. This paper will review the functional roles of specific amino acids, such as glutamine and arginine, in the development and maintenance of the GALT in the neonatal pig from birth through weaning. The neonatal gastrointestinal tract Both birth and weaning are associated with major changes in the development and metabolism of the gastrointestinal tract (GIT) and the GALT [4-9]. Prenatally the GALT is immature but after birth the GALT begins to develop rapidly, although some processes may take up to 6 weeks to mature. In the natural state piglets would be weaned, gradually, at 1012 weeks of age but in modern pig rearing this has been replaced by abrupt weaning at a much earlier (3-5 weeks) age that inflicts considerable stress on the entire gastrointestinal tract. Birth and suckling are associated with the initial introduction of microbes into the sterile GIT of the newborn. Since the pig placenta does not transfer immunoglobulins the consumption of colostrum represents the initial transfer of immunity to the neonate during the first two days before intestinal closure occurs [6]. During suckling the piglet is consuming a highly palatable, high fat milk diet that is relatively easy to digest. At weaning there is another major change in that the diet becomes relatively high in carbohydrate and more complex, which is not only more difficult to digest it is also associated with a dramatic change both in the number and variety of ingested microbes. Such early weaning is associated with low food intake and it may take up to two weeks for the piglet to regain energy intake similar to pre-weaning levels [7]. During this time there is a loss of body weight, excessive diarrhea, and lower feed efficiency. It has been estimated that at such time piglets achieve less than 50% of their growth performance. These problems are associated with major morphological changes in the structure of the small intestine such as decreased villus height and overall loss of function. At the same VI Congresso Latino-Americano de Nutrição Animal - SALA SUÍNOS 23 a 26 de Setembro de 2014 – Estância de São Pedro, SP - Brasil Realização: Colégio Brasileiro de Nutrição Animal - CBNA time it is critical to maintain the GALT to avoid undue systemic infections and to limit the incidence of diarrhea, all of which are occurring at a time that the GALT has not fully developed. Ultimately the GALT becomes the largest immune organ in the body and it is well established that protein deficiency is associated with immune deficiency and specific amino acids have major effects on both gut integrity and GALT function [4]. Various procedures have been proposed to deal with the problems of early weaning, many based on the addition of dietary supplements such as probiotics, prebiotics, individual small compounds such as amino acids, various preparations of plasma and other proteins, and more complex food constituents [6]. It is unlikely to think that one isolated supplement will cure all the ills of early weaning but adding back specific functional amino acids does show some promise, and at the same time avoids the need to feed high protein diets. Some studies have focused on supplementation of traditionally essential amino acids to a low protein diet which allows good growth and effectively lowers the incidence of diarrhea in abruptly weaned piglets [10]. This paper will consider other functional amino acids and how they may be useful in the development and maintenance of GIT and GALT function from birth through post-weaning. Functional Amino Acids in Neonatal Diets Glutamine Glutamine and glutamate represent the most abundant amino acids in milk and most commercial feedstuffs [11,12]. Often these two amino acids are expressed simply as “glutamate” since acid hydrolysis of proteins (used to determine specific amino acid content) effectively hydrolyses all glutamine to glutamate and thus the final estimate of “glutamate” in feedstuffs usually refers to the sum of glutamate plus glutamine. This is of little consequence since dietary glutamate and glutamine are both extensively catabolized within the small intestinal mucosa with only minor differences in their final end products [13]. Within the body glutamine is also the most abundant free alpha amino acid representing a pool of about 80g in the adult human, most of which is held within skeletal muscle cells [14,15]. Similarly, glutamine is the most abundant alpha amino acid in blood plasma of most mammals studied to date. In contrast, while intracellular glutamate concentrations are relatively high in most cells, the free glutamate concentration in plasma is maintained at a much lower concentration. Thus glutamine serves as a major transport form of nitrogen, carbon and energy between tissues, while glutamate plays a more direct role in intracellular intermediary metabolism. The major site of glutamine utilization of circulating glutamine is the small intestine where glutamine serves as the major respiratory substrate for the epithelial cells (enterocytes). In addition, after food consumption, enterocytes will use both glutamine and glutamate derived from the diet as their major fuels and effectively these two amino acids do not enter the circulation [13,14]. Enterocyte glutamine and glutamate metabolism also give rises to citrulline, alanine, proline, lactate, glutathione and ammonia. Of note, are the synthesis of glutathione, a major determinant in redox status, and of citrulline that is directly related to arginine synthesis as detailed below. In addition, glutamine is required for the synthesis of NAD, NADP, purines, pyrimidines, and glucosamine, all substances required by differentiating and growing cells, and these functions are probably the reason that glutamate cannot completely replace the use of VI Congresso Latino-Americano de Nutrição Animal - SALA SUÍNOS 23 a 26 de Setembro de 2014 – Estância de São Pedro, SP - Brasil Realização: Colégio Brasileiro de Nutrição Animal - CBNA glutamine by enterocytes and other cells. Similarly, glutamine is the major respiratory fuel for immune cells (macrophages, B and T lymphocytes, neutrophils, granulocytes) and anything that stimulates these cells to proliferate, mature, produce cytokines or undergo phagocytosis (macrophages) stimulates the utilization of glutamine [16,17]. Indeed, the survival of such cells in culture is highly dependent on high rates of glutamine utilization. As with enterocytes, while some glutamine is used for biosynthetic purposes involved in cell proliferation and differentiation the bulk is used as respiratory fuel. More recently it has been noted that in addition to its metabolic roles, glutamine has been shown to exhibit specific signaling properties including changes expression of key genes encoding metabolic and cryoprotective processes (e.g. heat shock proteins), and acting overall in an anabolic sense (increased proliferation and differentiation, increased protein synthesis, decreased proteolysis, decreased apoptosis) in most cells [18]. Thus glutamine and glutamate play essential, and quantitatively important, roles in the metabolism and function of both the epithelial barrier of the GIT and of the GALT in general. In catabolic states (burn, infections, etc.) there a release of glutamine from skeletal muscle and a decrease in the free glutamine pool within that tissue. Concomitantly there is an increase in muscle net proteolysis that provides the substrate for continued synthesis and release of glutamine to fuel immune cells, aid in wound repair, correct metabolic acidosis, and provide the carbon substrate for both hepatic and renal gluconeogenesis [15,16]. Over the past twenty years attempts to avoid such muscle loss in human clinical work has resulted in the use of large amounts (up to 40g per day) of glutamine provided by parenteral delivery. In addition, enteral glutamine has become a widespread dietary supplement in both clinical and recreational (body building) subjects. The benefits of enteral glutamine have mainly been the preservation of intestinal function in severely ill patients particularly in those receiving total parenteral nutrition. The use of supplemental glutamine/glutamate in the domestic animal industry is limited but a number of studies do support its use in development, particularly from birth through post-weaning and there is some evidence that it may also be beneficial to the mother to prevent the loss of lean body mass that accompanies lactation. The first study of glutamine supplementation [19] in piglets demonstrated that 1% glutamine preserved jejunal villus height after weaning and that there was a 25% increase in growth performance (gain:feed ratio) in the 14 days post weaning. Similarly, Zou et al. [20] found 28% faster growth, improved food conversion and reduction in the incidence of diarrhea with 1% glutamine. Hanczakowska and Niwinska [21] have reviewed the use of glutamine in pig nutrition and cite their own, unpublished, work where there was an increase in weight gain (8.7%) in piglets receiving 2% glutamine. They do however, note that not all studies show changes in weight piglet weight gain. This may be related many studies being terminated just a few days after weaning, before benefits to the piglet, such as improved weight gain or decreased incidence of diarrhea, would become apparent. Studies from Field and colleagues [22,23] also showed that glutamine (4.4%) could prevent the drop in skeletal muscle glutamine content in recently weaned piglets, had beneficial effects on immune cells in mesenteric lymph nodes and could normalize lymphocyte function in E. coli infected piglets. More recently this group looked at the VI Congresso Latino-Americano de Nutrição Animal - SALA SUÍNOS 23 a 26 de Setembro de 2014 – Estância de São Pedro, SP - Brasil Realização: Colégio Brasileiro de Nutrição Animal - CBNA effect of glutamine (4.4%) on intestinal barrier function in E. coli infected piglets and found that glutamine attenuated catabolism, suppressed inflammation and cytokine release, and maintained tight junction protein expression and epithelial barrier function [24]. Similarly, Yi et al. [25] found that glutamine (2%) could alleviate growth depression in E. coli infected piglets, in part by maintaining intestinal morphology and immune function, and possibly through maintaining growth hormone and IGF1 levels. Domeneghini and colleagues [26-28] showed that 0.5% glutamine increased villus height, decreased the number of apopotic cells and resulted in beneficial immunological changes in the intestine of weanling piglets. Overall, they found a positive effect on the barrier function, and an increase in epithelial cell number, and an increase in mitotic cells in epithelium. Wang et al. [29] used microarray analysis of gene expression in small intestine to investigate the molecular mechanisms responsible for the protective effects of glutamine in early weaning. They compared weaning at day 21 to a commercial diet with or without glutamine, with sampling at day 28, with piglets that had been allowed to nurse through day 28. Early weaning resulted in a marked decrease in body weight gain, small intestinal weight, length and villus height, together with less glutathione and a more oxidized glutathione ratio. This was accompanied by increased expression of genes involved in oxidative stress, and immune activation but decreased expression of genes related to macronutrient metabolism and cell proliferation in the small intestine. When the piglets were weaned to a diet supplemented with 1% glutamine, there was some alleviation of the effects on body weight gain, and intestinal weight, villus height, glutathione levels and an overall enhancement of oxidative-defense capacity. Furthermore glutamine supplemented piglets showed increased intestinal expression of genes involved in cell growth and removal of oxidants, and decreased expression of genes coding for factors that promote oxidative stress and immune activation. Zhong et al. [30] also looked at mechanisms in 21 day weaned piglets fed with 1g glutamine per kg body weight at 12 hour intervals for up to 14 days. Glutamine treatment increased average daily weight gain, lowered the incidence of diarrhea, increased the weight of small intestine and villus height, and increased expression of heat shock protein 70 (HSP70) mRNA and protein. HSP70 is believed to be involved in maintaining cell structure and function during stress and has been shown to have cytoprotective roles in epithelial function. Recently a number of studies from Xiao and colleagues [31-33] have investigated metabolomic changes in piglet intestine in response to glutamine (1%) supplementation, and while full details of these data sets are beyond the scope of this text, the findings point to important changes in luminal digestion of feedstuffs and in enterocyte intermediary metabolism. A few studies have been published using supplementation with glutamate or a mixture of glutamine and glutamate. Ewtushik et al. [34] found that glutamate (6.51%) prevented weaning-induced villus atrophy and enhanced both total and mucosal growth in the small intestine. Renazi et al. [35] used glutamate (up to 4%) supplementation for 3 weeks in early weaned piglets and found improved feed efficiency, reduced diarrhea, improved intestinal morphology, villus height, DNA content and antioxidant capacity. Similarly, Wu et al. [36] found that glutamate (1%) improved intestinal villus height and epithelial proliferation and morphology. Thus glutamate seems to have, at least some, of the potential as glutamine. VI Congresso Latino-Americano de Nutrição Animal - SALA SUÍNOS 23 a 26 de Setembro de 2014 – Estância de São Pedro, SP - Brasil Realização: Colégio Brasileiro de Nutrição Animal - CBNA Although glutamine/glutamate concentrations are high in milk it appears that these maybe insufficient for maximal growth of the piglets. Haynes and colleagues [37] fed glutamine or alanyl-glutamine by gavage two times a day for 7 days (from age 7 day) to piglets that were still nursing on the sow. The piglets then received injections of E. coli endotoxin and were sampled 24 and 48 h later. They found that extra glutamine decreased expression of Toll like receptor 4, active caspase 3 and NFκb (all increased in response to endotoxin), with an overall amelioration of intestinal injury, a decrease in rectal temperature, and enhanced piglet growth performance. Jiang et al. [38] used very early, day 14, weaning in piglets that were repeatedly (day 7, 14 and 21) challenged with endotoxin. In piglets receiving a glycyl-glutamine supplement they found improved growth and intestinal morphology in both control and LPS challenged piglets. Although the results of these studies indicate a potential role for glutamine supplementation prior to weaning treating individual piglets by twice daily gavage is not practical in a commercial pig rearing facility. An alternative approach is to use creep feeders as was done by Cabrera and co-workers [39]. They found that provision of glutamine (1%), or a mixture of glutamine plus glutamate (0.88% Aminogut), provided in creep feeders from days 14 to 21, resulted in improved intestinal villus height and feed conversion through six weeks of age. An alternative to individually feeding suckling piglets is to provide the glutamine supplement to the lactating sow with the aim of increasing milk glutamine content. We, and others have shown that lactation in pigs, and other species, is accompanied by a mild catabolic state as indicated by a loss of lean body mass and a decrease in intramuscular glutamine content [40] and thus supplemental glutamine to the sow may alleviate such losses. Kitt et al. [41] found that feeding 2.5% glutamine to sows increased glutamine content of milk and we saw similar results with glutamine (2.5%) or a mixture of glutamine and glutamate (2.5%) [40]. In both studies there were no changes in piglet performance. We did however find maintenance of skeletal muscle glutamine content in the sows that received the glutamine and or glutamine plus glutamate supplements, indicating some limitation in the catabolic response to lactation. Thus glutamine and/or glutamate supplementation to early weaned, suckling (creep feeders), or the lactating sow, may improve intestinal health and immune function, and so alleviate some of the stress of abrupt early weaning. Beneficial effects are seen with a little as 1% glutamine or glutamate but higher amounts do not seem to cause any problems in weaned piglets. Arginine Arginine is a basic amino acid that is recognized as being essential in the neonate for most mammalian species but is usually considered non-essential in adult mammals [4244]. Exceptions are carnivores such as cats and ferrets that are unable to synthesize arginine, apart from that used in the hepatic urea cycle, and thus have an absolute requirement for dietary arginine throughout life. There is however, evidence that dietary arginine deficiency impairs fertility in both humans and boars [44]. Although large amounts of arginine synthesis and degradation occur in the liver for urea synthesis, this arginine is not released from the liver and can be ignored with regard to extra-hepatic arginine metabolism. Arginine synthesis occurs through a two tissue pathway whereby in VI Congresso Latino-Americano de Nutrição Animal - SALA SUÍNOS 23 a 26 de Setembro de 2014 – Estância de São Pedro, SP - Brasil Realização: Colégio Brasileiro de Nutrição Animal - CBNA the intestine glutamate, glutamine or proline, are used to synthesize citrulline that is released into the circulation and taken up by the kidney where it is converted to arginine. In the neonatal period there is evidence that the piglet intestine can carry out the complete pathway to arginine but this has no bearing on the fate of arginine in the body [44]. Arginine (and citrulline), from the diet and that synthesized in the intestine and kidney, are not taken up by the liver (and thus not used for urea synthesis) and are available for extrahepatic functions. Arginine, through the action of nitric oxide synthase, is as the only precursor of nitric oxide which has important roles in both vasodilation and immune cell killing of pathogenic microbes. Thus it is not surprising that arginine is essential for the function of the GALT. In addition to its roles as a substrate for nitric oxide and protein synthesis, arginine is a precursor for the synthesis of creatine, proline, glutamate, and agmatine, and also a signal via mTOR to increase protein synthesis, decrease proteolysis. Similarly arginine stimulates the proliferation of enterocytes while decreasing apoptosis, and stimulates expression of HSP70 for cryoprotective roles in times of stress. Arginine is also a powerful stimulator of both insulin and growth hormone secretion, and positively regulates expression of genes related to growth and mitochondrial proliferation [18, 42-44]. Thus, in a similar manner to glutamine, arginine acts in an overall anabolic nature with respect to the GIT and GALT. Leibholz [45], using arginine supplementation to very early (day 3-4) weaned piglets reported that only 55% of arginine could be synthesized endogenously thus arginine was essential in such young piglets. Although arginine is relatively high in most proteins found in feedstuffs (usually 3-5% of the amino acids) it is, of course low in low protein components such as grains [12]. Surprisingly the arginine content in porcine milk is low and actually deceases as lactation progresses [11,37]. Equally surprising is the fact that, the endogenous pathway of arginine synthesis is poorly developed in neonatal piglets [44]. In part this is due to a lack of N-acetyl glutamate an obligate activator of the carbamoyl phosphate synthase 1 (CPS1) reaction in the intestinal pathway of citrulline production. This was clearly demonstrated by the finding that citrulline could replace arginine in the neonatal pig diet whereas proline could not [46]. Furthermore Wu and colleagues [36,47] used N-carbamoylglutamate (NCG), also acts to stimulate CPS1, to stimulate intestinal citrulline/arginine production in early weaned piglets. Comparing a control (no supplement) diet to those containing either 0.6% arginine or 0.08% NCG they found that both supplements showed higher rates of body weight gain, heavier and longer small intestines, higher villus height, increased expression of HSP70, and an increase in the number of goblet cells, all of which resulted in a decrease in the incidence of diarrhea. Kim and Wu have reported that arginine deficiency resulted in growth retardation, intestinal, reproductive and immune dysfunction, impaired neurological development, impaired wound healing, and high circulating ammonia concentrations [42,44]. In 2004 they reported that 0.2 to 0.4% arginine supplementation to very early (day 7) weaned piglets lowered plasma ammonia and enhanced body weight gain, unequivocally showing that arginine is limiting in suckling piglets [48]. More recently they reported that feeding 1% arginine to sows from day 30 before parturition through weaning, resulted in increased body weight gain in piglets through day 7 and day 21 after birth [49]. In their original paper [48] they reported that arginine supplementation increased circulating insulin and VI Congresso Latino-Americano de Nutrição Animal - SALA SUÍNOS 23 a 26 de Setembro de 2014 – Estância de São Pedro, SP - Brasil Realização: Colégio Brasileiro de Nutrição Animal - CBNA growth hormone levels and in more recent work they have extensively investigated the mechanisms involved in the beneficial effects of arginine in neonatal piglets. Zhan et al. [50] provided 0.7 or 1.2 % supplemental arginine to day 14 weaned piglets and found reduced incidence of diarrhea, but no difference in weight gain or food intake. They did however, find increased intestinal expression of CD38, VEGF, and jejunal endothelin-1, all indicators of enhanced microvascular development, in the arginine supplemented piglets. Likewise, Tan et al [51] using very early weaning at 7 days supplemented with 0 to 0.8% arginine found increased growth performance together with higher levels of IgG and IgM, but lower inflammatory cytokines lower, cellular, demonstrating that both cellular and humoral immunity were improved. Further evidence that arginine increases vascularization and intestinal morphology was provided by Yao et al. [52] who, using early weaned pigs supplemented with arginine (1%) found increased intestinal expression of VEGF, increased villus height, and higher plasma arginine and insulin, but lower ammonia and cortisol. Zheng and co-workers [53] using diquat to induce oxidative stress in day 28 piglets, showed that 0.8 or 1.8% arginine alleviated the negative effects of oxidative stress on on feed intake and significantly increased total antioxidant capacity and decreased expression of inflammatory cytokines. Similarly, Li et al. [54] reported that arginine (0.5%) supplementation to early weaned pigs alleviates morphological and immunological liver injury caused by E. coli endotoxin injection, in part through the action of nitric oxide. Further evidence that arginine acts in a cytoprotective manner was provided by Wu et al [55] who demonstrated that HSP70 expression was increased in the liver of arginine (6g/kg feed, three times a day) fed early weaned pigs and this was accompanied by decreased expression of inflammatory cytokines. There are also indications that arginine supplementation, possibly through nitric oxide formation and improved vascularization, improves reproductive performance with more piglets born alive, heavier litter weights, etc. [44]. The optimal amount of arginine supplementation for pigs seems to be between 0.5-1.0% of the diet. Some studies have reported adverse effects at higher concentrations which may be related to inappropriate arginine:lysine ratios resulting in amino acid imbalance and/or antagonism in absorption from the gut [43]. Other Amino Acids Supplementation with other individual amino acids have received little attention although there is some evidence that threonine, through its role as an essential precursor for mucin (barrier function) production is hypothesized to have potential benefits in maintaining GIT and GALT function. Similarly, methionine (and cysteine) through their roles in taurine and glutathionine production may also be beneficial to the GALT but no direct studies have been published. Interestingly, two very recent studies [56,57] have reported beneficial effects of aspartate supplementation in weanling piglets, but this may simply be related to the similarity of aspartate metabolism to glutamate metabolism within the enterocyte. Other amino acids such as glycine and proline have been hypothesized to be beneficial to the GALT but, to date, there are no reports of their use. VI Congresso Latino-Americano de Nutrição Animal - SALA SUÍNOS 23 a 26 de Setembro de 2014 – Estância de São Pedro, SP - Brasil Realização: Colégio Brasileiro de Nutrição Animal - CBNA Conclusions As we become more aware of important non-protein roles of amino acids there is clearly a need to re-classify some traditionally non-essential amino acids as “functional” amino acids. With regard to the maintenance GIT and GALT function, glutamine (glutamate) and arginine have been proven to be beneficial. They are required in the diet in sufficient amounts to achieve optimal performance in both early weaned piglets and possibly for the sow to maintain lean body mass during lactation. The focus of future studies should be to determine the optimal amount of the supplement, whether to give a single or multiple supplements, the role of the basal diet, the animal (sow or piglet) to be treated, and the time and duration of treatment. An important question is, what outcomes should be considered? Weight gain and feed efficiency are clearly important in the longterm but experimental studies will require suitable markers if we are to fully understand both the benefits, and the mechanisms of action, of such supplements. References 1. Rose, W.C. (1957) The amino acid requirements of adult man. Nutr. Abst. Rev. 27, 631-647 2. Wu, G. (2010) Functional amino acids in growth, reproduction and health. Adv. Nutr. 1, 31-37 3. Wu, G. (2013) Functional amino acids in nutrition and health. Amino Acids 45, 407-411 4. Ruth, M.R. and Field, C.J. (2013) The immune modifying effects of amino acids on gutassociated lymphoid tissue. J. Animal. Sci. Biotech. 4.27 doi10.1186/2049-1891-4-27 5. Bailey, M., Haverson, K., Inman, C., Harris, P., Jones, P., Corfield, G., Miller, B. and Stokes, C. (2005) The development of the mucosal immune system pre- and post-weanin: balancing regulatory and effector function. Proc. Nutr. Soc. 64, 451-457 6. Lalles, J.-P., Bosi, P., Smidt, H. and Stokes, C.R. (2007) Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 66, 260-268 7. Campbell, J.M., Crenshaw, J.D. and Polo, J. (2013) The biological stress of early weaning piglets. J. Animal. Sci. Biotech. 4:19 doi: 10.1186/2049-1891-4-19 8. Pluske, J.R. (2013) Feed- and feed additives-related aspects of gut health and developments in weanling pigs. J. Anim. Sci. Biotech. 4:1 doi: 10.1186/2049-1891-4-1 9. Jacobi, S.K. and Odle, J. (2012) Nutritional factors influencing intestinal health of the neonate. Adv. Nutr. 3, 687-696 10. Lordelo, M.M., Gaspar, A.M., Le Bellego, L. and Freire, J.P.B. (2008) Isoleucine and valine supplementation of a low-protein corn-wheat-soybean meal-based diet for piglets: Growth performance and nitrogen balance. J. Anim. Sci. 86, 2936-2941 11. Wu, G. and Knabe, D.A. (1994) Free and protein bound amino acids in sow’s colostrum and milk. J. Nutr. 124, 415-424 12. Li, X., Renzaei, R., Li, P. and Wu, G. (2011) Composition of amino acids in feed ingredients for animal diets. Amino Acids 40, 1159-1168 VI Congresso Latino-Americano de Nutrição Animal - SALA SUÍNOS 23 a 26 de Setembro de 2014 – Estância de São Pedro, SP - Brasil Realização: Colégio Brasileiro de Nutrição Animal - CBNA 13. 82 Watford, M. and Reeds, P.J. (2003) Glutamate metabolism in the gut. Forum. Nutr. 56, 81- 14. Curthoys, N.P. and Watford, M. (1995) Regulation of Glutaminase Expression and Glutamine Metabolism. Ann. Rev. Nutr. 15: 133-159 15. Watford, M. (2008) Glutamine metabolism and function in relation to proline synthesis and the safety of glutamine and proline supplementation. J. Nutr. 138: 2003S-2007S 16. Calder, P.C. and Yaqoob, P. (1999) Glutamine and the immune system. Amino Acids 17, 227-241 17. Li, P., Yin, Y., Li, D., Kim, S.W. and Wu, G. (2007) Amino acids and immune function. Brit. J. Nutr. 98, 237-252 18. Rhoads, J.M. and Wu, G. Glutamine, arginine and leucine signaling in the intestine. Amino Acids 37, 111-122 19. Wu, G., Meier, S.A. and Knabe, D.A. (1996) Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J. Nutr. 126, 2578-2584 20. Zou, X.T., Zheng, G.H., Fang, X.J. and Jiang, J.F. (2006) Effects of glutamine on growth performance of weanling piglets. Czech. J. Anim. Sci. 51, 444-448 21. Hanczakowska, E. and Niwinska, B. (2013) Glutamine as a feed supplement for piglets: A review. Ann. Anim. Sci. 13, 5-15 22. Yoo, S.S., Field, C.J., McBurney, M.I. (1997) Glutamine supplementation maintains intramuscular glutamine concentrations and normalizes lymphocyte function in infected early weaned pigs. J. Nutr. 127, 2253-2259 23. Johnson, I.R., Ball, R.O., Baracos, V.E. and Field, C.J. (2006) Glutamine supplementation influences immune development in the newly weaned piglet. Develop. Comp. Immunol. 30, 11911201 24. Ewaschuk, J.B., Murdoch, G.K., Johnson, I.B., Madsen, K.L. and Field, C.J. (2011) Glutamine supplementation improves intestinal barrier function in a weaned piglet model of Escherichia coli infection. Brit. J. Nutr. 106, 870-877 25. Yi, G.F., Carroll, J.A., Allee, G.L., Gaines, A.M., Kendall, D.C., Usry, J.L., Toride, Y. and Izuru, S. (2005) Effect of glutamine and spray-dried plasma on growth performance, small intestinal morphology, and immune responses of Escherichia coli K88+ - challenged weaned pigs. J. Animal. Sci. 83, 634-643 26. Domeneghini, C., Di Giancamillo, A., Arrighi, S. and Bosi, G. (2006) Gut-trophic feed additives and their effects upon the gut structure and intestinal metabolism. State of the art in the pig, and perspectives towards humans. Histol. Histopathol. 21, 273-283 27. Domeneghini, C., Di Giancamillo, A., Savoini, G., Paratte, R., Bontempo, V. and Dell’Orto, V. (2004) Structural patterns of swine ileal mucosa following L-glutamine and nucleotide administration during the weaning period. An histochemical and histometrical study. Histo. Histopathol. 19, 49-58 28. Domeneghini, C., Di Giancamillo, A., Bosi, G. and Arrighi, S. (2006) Can nutraceuticals after the structure of intestinal muscosa? Qualitative and quantitative microanatomy in L-glutamine diet-supplemented weaning piglets. Vet. Res. Comm. 30, 331-342 VI Congresso Latino-Americano de Nutrição Animal - SALA SUÍNOS 23 a 26 de Setembro de 2014 – Estância de São Pedro, SP - Brasil Realização: Colégio Brasileiro de Nutrição Animal - CBNA 29. Wang, J., Chen, L., Li, P., Li, X., Zhou, H., Wang, F., Li, D., Yin, Y. and Wu, G. (2008) Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J. Nutr. 138, 1025-1032 30. Zhong, X., Zhang, X.H., Li, X.M., Zhou, Y.M., Li, W., Huang, X.X., Zhang, L.L. and Wang, T. (2011) Intestinal growth and morphology is associated with the increase in heat shock protein 70 expression in weaning piglets through supplementation with glutamine. J. Anim. Sci. 89, 3634-3642 31. Xiao, Y., Wu, T., Hong, Q., Sun, J., Chen, A., Yang, C. and Li, X. (2012) Response to weaning and dietary L-glutamine supplementation metabolomics analysis in piglets by gas chromatography/mass spectrometry. J. Zhejiang Univ Sci B (Biomed and Biotechol) 13, 567-578 32. Xiao, Y.P., Wu, T.X., Sun, J.M., Yang, L., Hong, Q.H., Chen, A.G. and Yang, C.M. (2012) Response to dietary L-glutamine supplementation in weaned piglets: a serum metabolomics comparison and hepatic metabolic regulation analysis. J. Animal. Sci. 90, 4421-4430 33. Xiao, Y.P., Li, X.Y., Wu, T.X., Yang, L., Hong, Q.H., Yang, C.M., and Chen, A.C. (2012) Effects of dietary glutamine supplementation on nutrient absorption and activity of enzymes involved in glutamine metabolism and energy production in the jejunum of weaned piglets. J. Anim. Vet. Adv. 11, 1441-1449 34. Ewtushik, A.L., Bertolo, R.F.P. and Ball, R.O. (2000) Intestinal development of earlyweaned piglets receiving diets supplemented with selected amino acids or polyamines. Can. J. Animal. Sci. 80, 653-662 35. Renzaei, R., Knabe, D.A., Tekwe, C.D., Dahanayaka, S., Ficken, M.D., Fielder, S.E., Eide, S.J., Lovering, S.L. and Wu, G. (2013) Dietary supplementation with monosodium glutamate is safe improves growth performance in postweaning pigs. Amino Acids 44, 911-923 36. Wu, X., Zhang, Y., Liu, Z., Li, T.J. and Yin, Y.L. (2012) Effects of oral supplementation with glutamate or a combination of glutamate and N-carbamylglutamate mucosa morphology and epithelium cell proliferation in weanling piglets. J. Anim. Sci. 90, 337-339 37. Haynes, T.E., Li, P., Li, X., Shimotori, K., Sato, H., Flynn, N.E., Wang, J., Knabe, D.A. and Wu, G. (2009) L-Glutamine or L-alanyl-L-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids 37, 131-142 38. Jiang, Z.Y., Sun, L.H., Lin, Y.C., Ma, X.Y., Zheng, C.T., Zhou, G.L., Chen, F. and Zou, S.T. (2009) Effects of dietary glycyl-glutamine on growth performance, small intestinal integrity, and immune responses of weaning piglets challenged with lipopolysaccharide. J. Animal. Sci. 87, 4050-4056 39. Cabrera, R.A., Usry, J.L., Arrellano, C., Nogueira, E.T., Kutschenko, M., Moeser, A.J. and Odle, J. (2013) Effects of creep feeding and supplemental glutamine or glutamine plus glutamate (Aminogut) on pre- and post-weaning growth performance and intestinal health of piglets. J. Anim. Sci. Biotech. 4:29 doi:10 1186/2046-1891-4-29 40. Manso, H.E.C.C.C., Manso Filho, H.C., de Cavalho, L.E., Kutschenko, M., Nogueira, E. and Watford, M. (2012) J. Anim. Sci. Biotech. 3:2 doi:10 1186/2049-1891-3-2 41. Kitt, S.J., Miller, P.S. and Fischer, R.L. (2004) Effect of sow dietary glutamine supplementation on sow and litter performance, subsequent weanling pig performance and intestinal development after an immune challenge. Nebraska Swine Report 14-17 VI Congresso Latino-Americano de Nutrição Animal - SALA SUÍNOS 23 a 26 de Setembro de 2014 – Estância de São Pedro, SP - Brasil Realização: Colégio Brasileiro de Nutrição Animal - CBNA 42. Wu, G., Knabe, D.A. and Kim, S.W. (2004) Arginine nutrition in neonatal pigs. J. Nutr. 134, 2783S-2790S 43. Ball, R.O., Urschel, K.L. and Pencharz, P.B. (2007) Nutritional consequencesw of interspecies differences in arginine and lysine metabolism. J. Nutr. 137, 1626S-1641S 44. Wu, G., Bazer, F.W., Davis, T.A., Kim, S.W., Li, P., Rhoads, J.M., Satterfield, M.C., Smith, S.B., Spencer, T.E. and Yin, Y. (2009) Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37, 153-168 45. Leibholz, J. (1982) Arginine requirements of pigs between 7 and 28 days of age. Aust. J. Agric. Res. 33, 165-170 46. Urschel, K.L., Shoveller, A.K., Uwiera, R.R.E., Pencharz, P.B. and Ball, R.O. (2006) Citrulline is an effective arginine precursor in enterally fed neonatal piglets. J. Nutr. 136, 18061813 47. Wu, X., Ruan, Z., Gao, Y., Yin, Y., Zhou, X., Wang, L., Geng, M., Hou, Y. and Wu, G. (2010) Dietary supplementation with L-arginine or N-carbamylglutamate enhance intestinal growth and heat shock protein-70 expression in weanling pigs fed a corn- and soybean meal-based diet. Amino Acids 39, 831-839 48. Kim, S.W., McPherson, R.L. and Wu, G. (2004) Dietary arginine supplementation enhances the growth of milk-fed young pigs. J. Nutr. 134, 625-630 49. Mateo, R.D., Wu, G., Moon, H.K., Carroll, J.A. and Kim, S.W. (2007) Effects of dietary arginine supplementation during gestation and lactation on the performance of lactating primiparous sows and nursing piglets. J. Anim. Sci. 86, 827-835 50. Zhan, Z., Ou, D., Piao, X., Kim, S.W., Liu, Y. and Wang, J. (2008) Dietary arginine supplementation affects microvascular development in the small intestine of early-weaned pigs. J. Nutr. 138, 1304-1309 51. Tan, B., Li, X.G., Kong, X., Huang, R., Ruan, Z., Yao, K., Deng, Z., Xie, M., Shinzato, I., Yin, Y. and Wu, G. (2009) Dietary L-arginine supplementation enhances the immune status in early-weaned piglets. Amino Acids 37, 323-331 52. Yao, K., Guan, S., Li, T., Huang, R., Wu, G., Ruan, Z. and Yin, Y. (2011) Dietary Larginine supplementation enhances intestinal development and expression of vascular endothelial growth factor in weaning piglets. Brit. J. Nutr. 105, 703-709 53. Zheng, P., Yu, B., He, J., Tian, G., Luo, Y., Mao, X., Zhang, K., Che, L. and Chen, D. (2013) Protective effects of dietary arginine supplementation against oxidative stress in weaned piglets. Brit. J. Nutr. 109, 2253-2260 54. Li, Q., Liu, Y., Che, Z., Zhu, H., Meng, G., Hou, Y., Ding, B., Yin, Y. and Chen, F. (2012) Dietary L-arginine supplementation alleviates liver injury caused by Escherichia coli LPS in weaned pigs. Innate Immunity 18, 806-814 55. Wu, X., Xie, C., Yin, Y., Li, F., Li, T., Huang, R., Ruan, Z. and Deng, Z. (2013) Effect of L-arginine on HSP70 expression in liver in weaning piglets. BMC Vet. Res. 9.63 doi:10.1186/1746-6148-9-63 56. Leng, W., Liu, Y., Shi, H., Li, S., Zhu, H., Pi, D., Hou, Y. and Gong, J. (2014) Aspartate alleviates liver injury and regulates mRNA expressions of TLR4 and NOD signaling-related genes in weaned pigs after lipopolysaccharide challenge. J. Nutr. Biochem. 25, 592-599 VI Congresso Latino-Americano de Nutrição Animal - SALA SUÍNOS 23 a 26 de Setembro de 2014 – Estância de São Pedro, SP - Brasil Realização: Colégio Brasileiro de Nutrição Animal - CBNA 57. Pi, D., Liu, Y., Shi, H., Li, S., Odle, J., Lin, X., Zhu, H., Chen, F., Hou, Y. and Leng, W. (2014) Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J. Nutr. Biochem. 25, 456-462