* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 13
Survey
Document related concepts
Light-dependent reactions wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Biosequestration wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Transcript
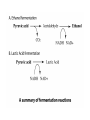
METABOLISM The net sum of all chemical reactions taking place in a cell! CATABOLISM • Breakdown/hydrolysis reactions • Usually liberate energy • We will study one example: – Glycolysis and Fermentation ANABOLISM • Synthesis/condensation reactions • Usually require energy • Examples include protein synthesis and DNA replication FOCUS ON: Carbohydrate Catabolism • Glucose is the most popular reactant, although other sugars will work. • Also, fats and proteins can be oxidized for energy, but we will not focus on them. • THE BEGINNING SET OF REACTIONS IS ALWAYS GLYCOLYSIS!!!!! Glycolysis in a nutshell • A molecule of glucose (6 carbons) is split into two molecules of pyruvic acid (3 carbons). This is an oxidation event. • A net gain of two ATP molecules is produced by substrate-level phosphorylation. • Two molecules of the coenzyme NAD+ are reduced to NADH. This is a reduction event. GLYCOLYSIS NEVER STANDS ALONE!!!! • After glycolysis, there are two choices: • Pyruvic acid may enter a fermentation pathway, or • It may enter a respiration pathway • Respiration may be aerobic or anaerobic; • Anaerobic respiration is not the same as fermentation!!!! (although fermentation requires no oxygen). The Fermentation Option: Lactic acid or ethanol • No ATP is produced during fermentation; why must these reactions take place? • NADH must be oxidized back to NAD+ so that glycolysis can continue!!!!!!!!! • Ethanol fermentation is most popular in microorganisms, especially yeast - beer, alcohol, bread, etc. Fermentation in a nutshell • Pyruvic acid is converted to – Lactic acid (3 carbons) OR – Ethanol and carbon dioxide • NO ATP is produced • NADH is oxidized to NAD+. Respiration involves • The Krebs Cycle (also called the TCA or citric acid cycle), and • A series of electron transfer agents (an electron transport chain), with an inorganic terminal electron acceptor (TEA). • If the TEA is oxygen, respiration is aerobic. • If the TEA is not oxygen, it is anaerobic (e.g., some bacteria can use nitrate or sulfate as the TEA.) Organisms can be grouped according to the way they obtain ENERGY and CARBON (two fundamentals of life!). We call this their nutritional pattern. • Heterotrophs - obtain carbon in an organic form and thus are dependent on other organisms. • Autotrophs - obtain carbon in inorganic form (carbon dioxide), and thus are not directly dependent on other organisms for their carbon needs. Organisms can be grouped according to the way they obtain ENERGY and CARBON (two fundamentals of life!). We call this their nutritional pattern. • Phototrophs - obtain energy from light; in other words, they are capable of photosynthesis. • Chemotrophs - obtain energy from the oxidation of chemical compounds. So, to get a more complete and accurate picture of an organism’s nutritional pattern, we can combine these terms: • Chemoheterotrophs - use reduced organic compounds for both carbon and energy. Most heterotrophs are of this type. Examples of this type include E.coli and human beings. • Chemoautotrophs - oxidize reduced inorganic compounds for energy (e. g. H2S, NH3, Fe), use CO2 as a carbon source. Examples include deep-sea vent bacteria and less exotic types such as many soil bacteria involved in decomposition and nutrient recycling. Also known as lithoautotrophs (“rock feeders”) So, to get a more complete and accurate picture of an organism’s nutritional pattern, we can combine these terms: • Photoautotrophs - use light as an energy source and CO2 as a carbon source. These are the most common ecological “producers” and include the cyanobacteria. • Photoheterotrophs - use light as an energy source and organic compounds for carbon. Examples include the green and purple nonsulfur bacteria.