* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Irradiated blood components
Survey
Document related concepts
Transcript
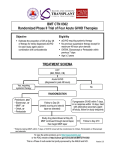
Indian J Med Res 122, November 2005, pp 371-373 Commentary Irradiated blood components The article by Agarwal et al1 in this issue focuses on storage lesions associated with irradiation of blood and introduces a special blood component for prevention of transfusion-associated graft versus-host-disease (TA- GVHD). TA-GVHD is under-reported and underdiagnosed because of its clinical similarity to viral infection, drug eruption or underlying disease. Precise incidence of transfusion induced GVHD is unknown but is higher than assumed. Recent recognition that TA-GVHD may occur not only in severely immunocompromised individuals but also in immunocompetent persons have broadened the spectrum of patient groups at risk for TA-GVHD2. This is explained by one way HLA match in which donor cells are homozygous for an HLA type for which the recipient is heterozygous. As a result, recipient immune cells do not recognize the donor cells as foreign and fail to mount an immune response that would normally clear the transfused donor cells through lymphocytolysis. Donor cells however, respond to mismatched haplotype and incite the graft versus host reaction which proceeds as aggressively as in immunocompromised patients. of lymphocytes. Foreign major histocompatibility (MHC) antigens or minor histocompatibility antigens of the host or both stimulate clonal T cell lymphocyte expansion with induction of an inflammatory response and cytokine release that is responsible for its clinical manifestation. GVHD was recognized in animals and was originally called ‘secondary’ or ‘runting’ syndrome. It followed the primary disease of radiation sickness induced in adult mice who had received subsequent spleen cell transplants. Grafted immunocompetent cells reacting against an immunodeficient neonatal host resulted in retarded growth, diarrhoea, skin lesions and liver abnormalities. It was Simonsen3 who suggested GVHD for this entity. The current concept of graft versus host reaction was first formulated in the 1950s when it was recognized that spleen transplants in irradiated mice initially resulted in recovery of animals from radiation injury and marrow aplasia but led to fatal secondary disease and ‘runting’ syndrome. In 1960s the term GVHD was coined and, Simonsen3 and Billingham4 defined minimum criteria for this condition. These criteria remain the basis for our understanding of the disease. One of the devastating effects of leucocytes present in blood components is TA-GVHD. It is mediated by immunologically competent lymphocytes in donated blood, which engraft, proliferate and mount a severe immunological response against host tissues. Likelihood of developing TA-GVHD is related to the number and viability of transfused lymphocytes in cellular blood components and extent of immunosuppression and degree of HLA antigen sharing between donor and recipient. Because treatment approaches to TA-GVHD have been largely ineffective, prevention is the only way out through complete elimination of mitotic potential Clinical GVHD was first described by Shimoda in 1955 as post-operative erythroderma in a case report of what was later identified as TA-GVHD. The first account of recognized TA- GVHD was published in mid 1960s by Hathaway and associates5. GVHD was first recognized in human in bone marrow transplant (BMT) recipients when histoincompatible marrow became functional and began to mount an immune reaction against its new host. Similar situation has been recognized in transfused patient groups when they 371 372 INDIAN J MED RES, NOVEMBER 2005 received blood products containing immunocompetent lymphocytes and patients were unable to prevent their grafting in them. Acute form of GVHD following transfusion occurs much more rapidly than that after BMT and does not respond to immunosuppression and hence much more fatal. Virtually all products that have not been stored are implicated in TA-GVHD. All of them contain varying amounts of viable lymphocytes averaging 1x107in a unit of whole blood or RBC. The dose of infused lymphocytes causing GVHD has ranged from 1x107/kg body weight in a neonate to 2x109/kg in a premature infant. Clinical experience with human T depleted marrow allograft has suggested that 104-105 clonable T cells/kg were sufficient to cause significant GVHD. There are currently no methods available to accomplish this degree of leucoreduction. Irradiation of blood components that completely eliminates lymphocyte mitotic potential remains the only practical way to avoid this. Irradiated cells though may seem morphologically and functionally intact, these cells lose their clonogenic potential and eventually die, either an inter- or intramitotic death. There are no adequate estimates of prevalence of TA-GVHD as most ‘at risk’ patients receive irradiated blood. On the basis of 1:102 homozygosity for one way human HLA haplotype sharing in Japan, transfusion of fresh rather stored red blood cells (RBCs) from first degree relatives, an estimated number of 40 cases of GVHD occur annually in Japan6. Recommendations for irradiation are much broader in Japan because of population homogeneity in HLA types. Spectrum of individuals at risk is likely to grow as intensive immunomodulatory therapies expand beyond oncological diseases and transplantation. Ionizing radiation can inhibit lymphocyte blast formation and mitogenic activity. Higher dose of radiation is required to abolish both. While lymphocytes appear to be susceptible with respect to their immunologic function to moderate doses of ionizing radiation, other cells appear to be more insensitive. Dose of irradiation chosen should be optimal to provide lymphocyte inactivation with minimal damage to other blood cells. Red cells are very resistant to irradiation. A dose of 10000 cGys did not affect 51Cr labeled red cell survival compared to controls7. In most studies, platelets have been unaffected by 5000 cGy with normal in vivo survival. Adverse effects of irradiation on blood components include a moderate decrease in survival of RBCs and leakage of potassium from intracellular stores. Caution should be exercised in administering stored irradiated RBCs to recipients at risk for ill effects of hyperkalaemia. American Association of Blood Banks (AABB) recommends not storing red blood cells for more than 28 days after irradiation. Platelets do not appear to be affected by the radiation dose during five day storage8. Studies have indicated that doses of 500-1000 cGy are sufficient to abolish reactivity in mixed lymphocyte culture (MLC). Limiting dilution assay (LDA) is considered to be more sensitive than the conventional MLC assay. MLC techniques are sensitive to only 1- 2 log10 inhibition of lymphocyte proliferation. Recently an LDA has been developed that can detect up to 5 log10 reductions in lymphocyte proliferative capacity9. With this T cell response becomes undetectable only after exposure to at least 2500 cGy of functional and viability properties of irradiated cellular blood components. Most critical is the delivered dose, and dosimetry measurements should be performed to ensure accuracy in the delivered dose. Threshold dose of lymphocytes to incite TA-GVHD in humans is not known, but case reports suggest that leucoreduction by current filtration (1-5 x 10 6 white blood cells/unit of blood) is not sufficient to prevent this disease. A minimum dose of 107 WBC/kg appears to be necessary in animals. Patients have developed fatal GVHD after transfusion of as few as 104cells/kg10. Current available information suggests that exposure of blood components to at least 2500 cGy is sufficient to prevent GVHD. Food and Drug Administration (FDA) has indicated that recommended dose of 2500 cGy is to be delivered to the mid plane of blood component with a minimum dose of 1500 cGy delivered throughout the component11. Gamma irradiation prevents proliferation of lymphocytes through cross-linkage of DNA. The damaged lymphocytes are unable to proliferate in the host and hence cannot mediate TA- GVHD. On the basis of animal models and estimate from bone marrow transplant data, 5x104 to1x105 T cells/kg body weight in an ablated host induce GVHD and a greater number MATHAI: IRRADIATED BLOOD COMPONENTS is needed in a non ablated host. Almost all blood components contain substantial number of lymphocytes to induce GVHD in a susceptible host. It is generally accepted that the freezing and thawing processes destroy the T lymphocytes that are present in fresh frozen plasma. There could also be a cumulative effect of viable T cells present in multiple transfusions by a patient whose immunological status fluctuates with time from treatment modality and disease status. It is suggested that cytotoxic T lymphocytes (CTLs) or inter leukin-2 (IL-2) secreting precursors of helper T lymphocytes may be more predictive of GVHD than the number of T cells alone. Gamma irradiation of blood components containing viable lymphocytes is virtually 100 per cent effective, though rare failures have been reported with suboptimal doses12. Two types of ionizing radiation - gamma rays and X-rays are similar in inactivating T lymphocytes in blood components at a given absorbed dose. Gamma irradiation is done by Cesium 137 or Cobalt 60 encased in dedicated blood bank irradiators. X-rays are generated from the interaction of a beam of electrons with a metallic surface like linear accelerators used for patient therapy. Major concern about TA-GVHD is that it is associated with serious morbidity and mortality in 75-90 per cent of affected patients. Incidence of GVHD declines with greater degree of HLA polymorphism among population, lower level of dependence on fresh blood with conservative transfusion practices followed. There is a need for continued diligence and high index of suspicion when a patient with recent transfusion history presents with symptoms for detection of TA-GVHD. For the future, technologies which inactivate lymphocytes with cellular components such as viral inactivation may offer a potential alternative to prevent TA-GVHD. Photochemical treatment using psoralens and long wave length UV irradiation (UVA) is under efficacy trials13,14. Jaisy Mathai Division of Blood Transfusion Services Sree Chitra Tirunal Institute for Medical Sciences & Technology (SCTIMST) Thiruvananthapuram 695011, India e-mail: [email protected] 373 References 1. Agarwal P, Ray VL, Choudhury N, Choudhury RK. Effect of pre-storage gamma irradiation on red blood cells. Indian J Med Res 2005; 122 : 385-7. 2. Anderson K. Broadening the spectrum of patient groups at risk for transfusion-associated GVHD: implications for universal irradiation of cellular blood components. Transfusion 2003; 43 : 1652-4. 3. Simonsen M. Graft versus host reactions. Their natural history, and applicability as tools of research. Prog Allergy 1962; 6 : 349-467. 4. Billingham RE. The biology of graft-versus-host reactions. Harvey Lect 1966; 67 : 21-78. 5. Hathaway WE, Fulginiti VA, Pierce CW, Githens JH, Pearlman DS, Muschenheim F, et al. Graft-vs-host reaction following a single blood transfusion. JAMA 1967; 201 : 1015-20. 6. Ohto H, Anderson KC. Posttransfusion graft-versus-host disease in Japanese newborns. Transfusion 1996; 36 : 117-23. 7. Holland PV. Transfusion associated graft versus host disease: Prevention using irradiated blood products. In: Garatty G, editors. Current concepts in transfusion therapy. Arlington VA: American Association Blood Banks; 1985 p. 295-316. 8. Read EJ, Kodis C, Carter CS, Leitman SF. Viability of platelets following storage in the irradiated state. A paired-controlled study. Transfusion 1988; 28 : 446-50. 9. Moroff G, Leitman SF, Luban NL. Principles of blood irradiation, dose validation, and quality control. Transfusion 1997; 37 : 1084-92. 10. Akahoshi M, Takanashi M, Masuda M, Yamashita H, Hidano A, Hasegawa K, et al. A case of transfusion-associated graft-versus-host disease not prevented by white cell-reduction filters. Transfusion 1992; 32 : 169-72. 11. Leitman SF. Dose, dosimetry, and quality improvement of irradiated blood components. Transfusion 1993; 33 : 447-9. 12. Luban NLC, Wong ECC. Irradiated products and washed/ volumed reduced products. In: Blood banking and transfusion medicine. Basic principles and practice. Hillyer CD, Silberstein LE, Ness PM, Anderson KC, editors. Philadelphia: Churchill Livingstone; 2003 p. 253-84. 13. Van Prooijen HC, Aarts-Riemens MI, Grijzenhout MA, Van Weelden H. Ultra violet irradiation modulates MHC-alloreactive cytotoxic T-cell precursors involved in the onset of graft-versus-host disease. Br J Haematol 1992; 81 : 73-6. 14. Grass JA, Wafa T, Reames A, Wages D, Corash L, Ferrara JL, et al. Prevention of transfusion-associated graft-versus-host disease by photochemical treatment. Blood 1999; 93 : 3140-7.