* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Developmental Neurotoxicity from Environmental Chemical Exposures
Survey
Document related concepts
Transcript
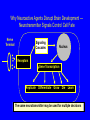
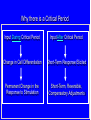
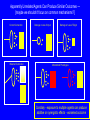
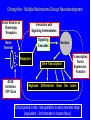
Drug and Chemical Exposures in Animal Models Related to ASD Theodore Slotkin, Ph.D. Department of Pharmacology & Cancer Biology Integrated Toxicology & Environmental Health Program Duke University Support: NIH ES10356 Main Points • Why an increase in neurodevelopmental disorders including ASD? • Why do neuroactive agents produce permanent alterations with developmental exposures? • Why is there a critical period for these effects? • Why do apparently unrelated agents produce similar outcomes? • Example from environmental chemicals: organophosphate pesticides • Example from prenatal drug exposure: terbutaline Developmental Neurotoxicity from Environmental Chemical Exposures 5000 new chemicals/year EPA estimate: 25% neurotoxic 67% of High Production Chemicals Not Tested for Neurotoxicity High vulnerability of the developing brain Increases in ADHD, learning/cognitive problems? • 17% of US schoolchildren suffer from neurobehavioral disabilities • Annual cost: $80-170 billion • 250% increase in ADHD diagnosis between 1990-1998 • 190% increase in children in special ed for learning disabilities between 1977-1994 • Increase in autistic spectrum disorders from 4/10,000 (1980s) to 30-60 (1990s) Developmental Neurotoxicants - The “Silent Pandemic” LDDI Initiative, 2007 Grandjean & Landrigan, Lancet 2006 Why Neuroactive Agents Disrupt Brain Development — Neurotransmitter Signals Control Cell Fate Nerve Terminal Signaling Cascades Nucleus Receptors Gene Transcription Replicate Differentiate Grow Die Learn The same neurotransmitter may be used for multiple decisions Why there is a Critical Period Input During Critical Period Input After Critical Period Change in Cell Differentiation Short-Term Response Elicited Permanent Change in the Response to Stimulation Short-Term, Reversible, Compensatory Adjustments Apparently Unrelated Agents Can Produce Similar Outcomes — [maybe we shouldn’t focus on common mechanisms?] Correct Connection Miswired Connection Damage or Loss of Input Damage or Loss of Target Mismatched Phenotypes Corollary - exposure to multiple agents can produce additive or synergistic effects - worsened outcome Organophosphate Pesticides — Chlorpyrifos • Widely used - ubiquitous exposure - OPs = 50% of all insecticide use • Not an endocrine disruptor • Replaced organochlorines • Superfund Site Disposal Problem • OPs: nerve gases in warfare/terrorism Developmental neurotoxicity unrelated to mechanisms in adults Effects are subtle but widespread Originally modeled in animals, neurodevelopmental deficits now confirmed in children (inner-city, agricultural populations) Developmental exposure increases autism risk Chlorpyrifos - Multiple Mechanisms Disrupt Neurodevelopment Direct Actions on Cholinergic Receptors Interaction with Signaling Intermediates Signaling Cascades Nerve Terminal Nucleus Transcription Factor Expression, Function Receptors Gene Transcription AChE Inhibition: CPF Oxon Replicate Differentiate Grow Die Learn Critical period in rats: late gestation to early neonatal stage [equivalent - 2nd trimester in human fetus] Chlorpyrifos - Impact on Serotonin Systems = Miswiring Male Female Chlorpyrifos T reatment on PN1-4 — 1 mg/kg ANOVA: Rx, p < 0.0001; Rx x sex, p < 0.0002; Rx x region, p < 0.0001; Rx x measure, p < 0.0003; Rx x region x measure, p < 0.0007 50 male female percent change from control 40 30 Rx x sex, p < 0.00 4 Rx x mea sure, p < 0.005 Rx x mea sure, p < 0.09 * Rx x sex, p < 0.1 Rx x mea sure, p < 0.001 Rx, p < 0.002 Rx x sex, p < 0.0006 ma le: p < 0.00 04 fe male : NS Rx, p < 0.0001 Rx x mea sure, p < 0.006 * * * 20 * 10 * 0 -10 * -20 5HT 1A 5HT 2 5HTT 5HT 1A 5HT 2 5HTT 5HT 1A 5HT 2 5HTT 5HT 1A 5HT 2 5HTT 5HT 1A 5HT 2 5HTT cerebral cortex Enhanced neuronal impulse activity (serotonin turnover) hippocampus striatum midbrain brainstem Increases in serotonin receptors and transporter BUT…. …Impaired Serotonergic Function Plus Maze: CPF (1 mg/kg) Decreases Anxiety in Males Chocolate Milk Preference: CPF (1 mg/kg) Causes Anhedonia 25 7 Control CPF 6 * Milk/Water Preference Percent Time Spent in Open Arms 30 20 15 10 5 Control CPF 5 4 * * 3 2 1 0 0 Male Female aka: increased risk-taking, impulsive behavior Male Female Chlorpyrifos - Miswiring of Acetylcholine Systems Serotonin Replaces Acetylcholine for Hippocampal Circuits and Behaviors 12 Working Memory Errors 10 PN 1-4 Chlorpyrifos 5HT2 Antagonist Drug Challenge 0 mg/kg ketanserin 0.5 mg/kg ketanserin 1.0 mg/kg ketanserin 2.0 mg/kg ketanserin * 8 * * 6 4 2 0 Control Chlorpyrifos p < 0.0001 Terbutaline Use in Preterm Labor • Stimulates BARs to inhibit uterine contraction • Crosses the placenta to stimulate fetal BARs • Effective for 48 hr max - NOT for maintenance use • Animal studies from our lab, 1980s-1990s altered neural cell differentiation receptor and signaling shifts permanent changes in responsiveness • Hadders-Algra 1986 - impaired school performance • Pitzer 2001 - psychiatric, learning disorders Cerebellum Control Terbutaline 44% decrease in Purkinje cells Thinning of cerebellar lobules Thinning of hippocampal CA3 Reactive gliosis Somatosensory cortex - loss of pyramidal cells Critical Period Newborn Rat - PN2-5 = human 2nd trimester • Neuroinflammation in cerebral cortex and cerebellum - microglial activation • Morphological changes almost identical to those in postmortem autism samples • Critical period PN2-5 • Hyperreactive to novelty, aversive stimuli, sensory input Decompensation of CVS responses similar to those in autism (compare to Ming 2005) • Continuous terbutaline exposure for 2 weeks: RR=2.0 • Male twins with no other affected siblings: RR=4.4 Further increase: BAR polymorphisms (16G, 27E) that prevent desensitization and therefore would enhance terbutaline effects Terbutaline - Impact on Serotonin Systems = Miswiring ≈ Chlorpyrifos Enhanced neuronal impulse activity (serotonin turnover) Increases in serotonin receptors and transporter Terbutaline Followed by Chlorpyrifos Enhanced Effect on Serotonin Turnover CONCLUSIONS • Developmental neurotoxicants likely to play an important role in the increased incidence of childhood behavioral disorders including ASD • Disparate mechanisms and effects converge on common final pathways — different agents may produce similar outcomes — different agents may produce additive/synergistic outcomes • Lasting effects only when exposure occurs in critical periods • Specific examples with relevance to ASD: — organophosphate pesticides (ubiquitous exposure) — terbutaline (use in preterm labor ≈10% US pregnancies) Neurodevelopmental disorders - CAUSES, not a single ‘cause’ Origins of autism and ASD may not be so distinct from other neurodevelopmental disorders