* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download CHAPTER 21: ELECTRIC CHARGE AND ELECTRIC FIELD
Survey
Document related concepts
History of electromagnetic theory wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
Circular dichroism wikipedia , lookup
Work (physics) wikipedia , lookup
Magnetic monopole wikipedia , lookup
Anti-gravity wikipedia , lookup
Weightlessness wikipedia , lookup
Speed of gravity wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Maxwell's equations wikipedia , lookup
Fundamental interaction wikipedia , lookup
Electromagnetism wikipedia , lookup
Field (physics) wikipedia , lookup
Lorentz force wikipedia , lookup
Transcript
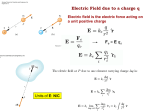
CHAPTER 21: ELECTRIC CHARGE AND ELECTRIC FIELD • As you may already know, according to our present understanding of the universe, there are four fundamental interactions, or forces: gravitation, electromagnetism, the weak interaction, and the strong interaction • You learned about gravitation in mechanics. This semester we will study another of these – electromagnetism • Electromagnetic interactions involve particles that have electric charge, which, like mass, is a fundamental property of particles • This chapter begins our foray into electrostatics – interactions between electric charges that are at rest (or at least to a good approximation they can be treated as such) • Specifically, this chapter will deal with three fundamental physical quantities in electromagnetism (by the way, I will often say “E and M” to refer to electromagnetism). Namely, charges and fields which give rise to forces. • The book presents two examples – rubbing glass with silk and rubbing plastic with fur – in order to introduce the concept of electric charge • The plastic rod rubbed with fur is associated with negative charge and the glass rod rubbed with silk is associated with positive charge • There is nothing unique about these materials – anytime two objects are rubbed together, odds are there will be some amount of charge transfer between them • Several important properties are readily deduced from experiments like these: 1) There are only two kinds of electric charge: positive and negative 2) Objects with “like charges” (charges with the same algebraic sign) exert a repulsive force on each other 3) Objects with “opposite charges” (charges with opposite algebraic signs) exert an attractive force on each other 4) If the objects do not exert a force on each other, we say that they are neutral 5) The strength of the electric force decreases as the distance between the objects increases 6) The greater the charge on the objects, the stronger the electric force is between them • An atom has a very dense and massive nucleus, which is positively charged since it contains positively charged protons and neutral neutrons • The nucleus is surrounded by the much-less-massive, negatively charged electrons • Electrons and protons are the basic charges in ordinary matter – they have charges of opposite sign but they are exactly equal in magnitude • Protons are extremely tightly bound within the nucleus, but electrons are bound much more loosely – so objects gain positive charge not by gaining protons, but by losing electrons • Ionization is the process of removing or gaining electrons • An atom that is missing electrons is called a positive ion and an atom that can accommodate extra electrons can become a negative ion • Most macroscopic objects have an equal number of protons and electrons – such objects are said to be electrically neutral • So when we speak of the charge of an object, we always mean its net (or excess) charge • Now back to charging objects through friction – nowhere in the process of rubbing two objects together did charge either appear or disappear • In fact, in any closed system, if a certain amount of positive charge appears somewhere, an equal amount of negative charge must appear somewhere else so that the net charge does not change • This is a fundamental principle known as the principle of conservation of charge: The algebraic sum of all the electric charges in any closed system is constant • In other words, charge is neither created nor destroyed, only transferred from one place to another • Charge is also observed to be quantized – electric charge is always an integer multiple of the elementary unit of charge, which is the amount of charge carried by a proton or an electron and has magnitude e = 1.60 x 10-19 C. C stands for Coulomb and is the SI unit for charge. • An insulator is a material in which the electrons are tightly bound to the positive nuclei and are not free to move around – although they can shift their positions somewhat • A conductor is a material in which the outer electrons (valence electrons) are weakly bound to the nuclei – in fact, they are detached from their parent nuclei and are free to wander through the entire solid, creating a highly mobile “sea of electrons” permeating an array of positively charged ion cores • We have already discussed charging an object through friction • Another way to charge an object is through conduction – this involves the direct physical contact of a charged object with a neutral object • Yet another way to charge a neutral object is through a process known as induction • It turns out that a charged object can even attract a neutral object • Consider, for example, the case of a positively charged object brought near (but not touching) a neutral conducting sphere • This is known as charge polarization, which is the separation of the positive and negative charges in a neutral object when a charged object is brought near it • The negative charges at the top of the sphere are closer to the charged rod than the positive charges at the bottom of the sphere. Thus, the attractive force between the sphere and the rod is stronger than the repulsive force, and therefore there is a net attractive force • This is called the polarization force – it arises because the charges in a neutral object are slightly separated, not because the two objects are oppositely charged • The polarization force between a charged object and a neutral object is always attractive, regardless of the charge COULOMB’S LAW • In words, this law states: the magnitude of the electrical force between two point charges is directly proportional to the magnitude of the product of the charges and inversely proportional to the square of the distance between them • Mathematically, this law is stated as: 𝐹𝐹 = 𝑘𝑘 |𝑞𝑞1 𝑞𝑞2 | 𝑟𝑟 2 • Note the similarity between this law of electrical force and the law of gravitational force. Like G, k is a constant of proportionality whose value depends on the system of units used. In SI units, k ≈ 9.0 x 109 N∙m2/C2. • The direction of the electric force lies along the line joining q1 and q2 • When the charges have the same sign, the forces are repulsive and when the charges have opposite signs, the forces are attractive • Like the law of gravitation, which only applies to point masses or masses that can be treated as such, Coulomb’s law only applies to point charges or charges that can be treated as such • Coulomb’s law is a force law and forces are vectors – they have both a magnitude and a direction • Additionally, electric forces, like ALL forces, can be superposed (principle of superposition). So, to get the net force on an object, you add, as vectors, the individual forces acting on it. • The net electric force on charge j due to all the other charges is the vector sum of the individual forces due to each charge (other than charge j): **EXAMPLES #1 and #2** • Before moving any further, I want to discuss a concept that we will use many times throughout the semester – the unit vector. You have already seen examples of these in mechanics: 𝒊𝒊̂ and 𝒋𝒋̂, unit vectors in the positive x and y directions, respectively. �: • First, let me rewrite Coulomb’s law in terms of the unit vector 𝒓𝒓 �𝑭𝑭⃗12 = 𝑘𝑘 𝑞𝑞1 𝑞𝑞2 𝒓𝒓� 𝑟𝑟 2 • �𝑭𝑭⃗12 is the force exerted by the charge 𝑞𝑞1 on the charge 𝑞𝑞2 . 𝒓𝒓� lies on the line passing through the two charges and points in the direction from 𝑞𝑞1 𝒓𝒓12 from toward 𝑞𝑞2 . That’s the same direction as the displacement vector �⃗ 𝑞𝑞1 to 𝑞𝑞2 except �⃗ 𝒓𝒓12 is not a unit vector – it does not have magnitude equal to 1 • It is quite easy to make it a unit vector, however. Simply divide the vector by its magnitude. Magnitude is a scalar so dividing by it won’t change the direction, it just changes its length, making it 1. • So in Coulomb’s law 𝒓𝒓� = �⃗12 𝒓𝒓 𝑟𝑟 where 𝑟𝑟 is the magnitude of �⃗ 𝒓𝒓12 , the distance between the charges • Alternatively, you can calculate the unit vector using trigonometry. Let 𝜃𝜃 �⃗12 makes with the positive x axis. Then the be the angle that the vector 𝒓𝒓 unit vector is: 𝒓𝒓� = cos 𝜃𝜃 𝒊𝒊̂ + sin 𝜃𝜃 𝒋𝒋̂ • Of course, you can always just find the magnitude of the force first, decide whether it is attractive or repulsive, and then calculate its components in whatever coordinate system you choose ELECTRIC FIELD AND ELECTRIC FORCES • The field model explains how a long-range force such as that due to charges is transmitted through empty space from one charge to another • Some kind of electric influence due to a charge fills the space around that charge whether or not there is a second charge there to feel the force that the first charge would exert on it • In the force model of the electric field, some charge A exerts a force on some other charge B • In the field model, it is the alteration of space around charge A that is the agent that mediates the force on charge B • This alteration of space is what we call a field • The space around a charge is altered to create an electric field just as the space around a mass is altered to create a gravitational field • When considering the force of gravity near the surface of the Earth, �⃗ 𝑭𝑭 �𝑭𝑭⃗ = 𝑚𝑚𝒈𝒈 ��⃗ or 𝒈𝒈 ��⃗ = . So we can think of ��⃗ 𝒈𝒈 as a measure of the force per 𝑚𝑚 unit mass (N/kg) that any object would experience if it were there. • This is the concept of the gravitational field • We will do the same with the electric force – we define the electric field �⃗ 𝑭𝑭 ��⃗ 𝑬𝑬 = as the force per unit charge, so it has units N/C 𝑞𝑞 • The field model describes how charges interact: 1. A group of charges, which we will call the source charges, alters the ��⃗ space around them and creates an electric field, 𝑬𝑬 2. If a point charge q0, which we will call the test charge or probe charge, is then placed in this electric field, it will experience a force �𝑭𝑭⃗ = 𝑞𝑞0 ��⃗ 𝑬𝑬 exerted by the field 3. The electric field, a vector field, exists at every point in space. Electric field diagrams will show a sample of vectors, but there is an electric field vector at every point whether one is shown or not. 4. If the probe charge q0 is positive, then the vector representing the force on q0 and the electric field vector are in the same direction; if q0 is negative, then they are in opposite directions 5. The electric field does not depend on the magnitude of the charge used to probe the field – it only depends on the source charges that create the field Electric Field of a Point Charge • Coulomb’s law gives the force between a point source charge q and some point test charge q0. Since q0 is a point charge, the electric field is simply given by ��⃗ 𝑬𝑬 = �𝑭𝑭⃗ 𝑞𝑞0 . So ��⃗ 𝑬𝑬 = 𝑘𝑘 𝑞𝑞 𝑟𝑟 2 𝒓𝒓� is the electric field due to a point charge q • It points radially outward, away from a positive charge and radially inward, towards a negative charge Electric Field Diagrams • An electric field diagram is a representative sample of electric field vectors due to some charge distribution, however the vector field exists at every point. A well-drawn diagram gives a good indication of what the field would be like everywhere in space. • The arrow indicates the direction and the strength of the electric field at the point to which it is attached – at the point where the tail of the vector is placed. The length of any vector is significant only relative to the lengths of other vectors. • Although we have to draw an arrow across the page, from one point to another, an electric field vector does not “stretch” from one point to another. Each arrow represents the electric field located at its tail. Calculating the Electric Field for More Complicated Source Charge Distributions • Since the electric force obeys the principle of superposition and the electric field is force per unit charge, then the electric field also obeys principle of superposition: �𝑬𝑬⃗ = 𝑬𝑬 �⃗1 + �𝑬𝑬⃗2 + �𝑬𝑬⃗3 + ⋯ = � 𝑬𝑬 �⃗𝑖𝑖 = � 𝑖𝑖 𝑖𝑖 𝑘𝑘𝑞𝑞𝑖𝑖 𝒓𝒓� 𝑟𝑟𝑖𝑖 2 𝑖𝑖 • This will form the basis of our main tool for calculating the electric field for continuous charge distributions as well, so let’s make sure we thoroughly understand each piece • The ��⃗ 𝑬𝑬𝑖𝑖 ’s are the fields of the point source charges 𝑞𝑞𝑖𝑖 located at distances 𝑟𝑟𝑖𝑖 from the point at which we are evaluating the field, and the 𝒓𝒓�𝑖𝑖 ’s are unit vectors pointing from each 𝑞𝑞𝑖𝑖 toward the point at which we are evaluating the field The Field of an Electric Dipole • One of the most important charge distributions is the electric dipole, consisting of two point charges of equal magnitude but opposite sign held a fixed distance apart • Many molecules can be modeled as a dipole and there numerous technological applications of the dipole configuration as well **SEE EXAMPLE #3** Continuous Charge Distributions • To calculate the field of a continuous charge distribution, we break up the region where the charge exists into many small charge elements dq and treat each as a point charge ��⃗ = 𝑘𝑘𝑘𝑘𝑘𝑘 • Each dq creates an electric field given by: 𝑑𝑑𝑬𝑬 𝒓𝒓� 2 𝑟𝑟 ��⃗’s and in the limit of infinitely • We then form the vector sum of all the 𝑑𝑑𝑬𝑬 ��⃗ = many dq’s the sum becomes an integral and the total field is: 𝑬𝑬 ��⃗ = ∫ 𝑘𝑘𝑘𝑘𝑘𝑘 𝒓𝒓� ∫ 𝑑𝑑𝑬𝑬 2 𝑟𝑟 • The limits of integration are chosen to include the entire source charge distribution ��⃗ separately – for • It is usually easier to calculate each component of 𝑬𝑬 example, 𝐸𝐸𝑦𝑦 = ∫ 𝑑𝑑𝐸𝐸𝑦𝑦 • An important step in setting up the integral is converting dq into something involving position. First, you will need to decide if the charge is distributed over a volume (3D), a surface (2D), or a line (1D). • Then use the appropriate charge density times an infinitesimal volume, area, or length element • ρ is the symbol for a volume charge density (C/m3); dq = ρdV • σ is the symbol for a surface charge density (C/m2); dq = σdA • λ is the symbol for a linear charge density (C/m); dq = λdx **SEE EXAMPLE #4, #5, and #6** ELECTRIC FIELD LINES • In addition to electric field diagrams, another way to visualize the electric field is to draw electric field lines • These are imaginary lines drawn through a region of space such that: 1. The tangent to a field line at any point is in the direction of the electric field ��⃗ 𝑬𝑬 at that point 2. The field lines are closer together (i.e., the field line density is larger) where the electric field strength is greater 3. The tangent to the field line indicates the direction of the force on a positive charge. The force must be in a unique, well-defined direction. Therefore, two field lines can never cross 4. The electric field is created by charges. A field line starts on a positive charge and ends on a negative charge (otherwise it extends to infinity). ELECTRIC DIPOLES • We did an example earlier where we calculated the field due to an electric dipole. Here we will assume that the charges +q and –q are separated by a distance d • Dipoles create their own electric field as we have seen, but what happens when you place a dipole in an electric field due to some other charge distribution? • Let’s place the dipole in a uniform electric field created by two charged parallel plates (see Example 21.12). • It is a uniform field so the magnitudes of the forces on the two charges of the dipole are equal. But the charges are of opposite sign, so the forces are in opposite directions and the net force on the dipole is zero. • In the figure below, the two forces both exert a clockwise torque about the midpoint, so their torques do not cancel • The lever arm for both forces is (dsinϕ)/2. The forces both have magnitude qE, so each exerts a torque (qE) (dsinϕ)/2. The torques are in the same direction so the net torque is: τ = (qE) (dsinϕ) • A quantity that describes the amount of polarization (charge separation) is called the electric dipole moment �𝒑𝒑⃗. It has magnitude p = qd and it points from the negative charge to the positive charge. • In terms of the dipole moment the torque due to a uniform field is τ = ��⃗. pEsinϕ, where ϕ is the angle between the vectors �𝒑𝒑⃗ and 𝑬𝑬 • Because of this we can express the torque exerted on a dipole by any electric �⃗ × ��⃗ �⃗ = 𝒑𝒑 field as a cross product: 𝝉𝝉 𝑬𝑬 ��⃗. So 𝜙𝜙 = 0 is a stable �⃗ in the direction of 𝑬𝑬 • The torque always tends to align 𝒑𝒑 �⃗ = 0). 𝝉𝝉 �⃗ = 0 when 𝜙𝜙 = 𝜋𝜋 as well, but this is an unstable equilibrium (𝝉𝝉 equilibrium. • Because there is a net torque acting over an angular displacement, it does work on the dipole. During an infinitesimal angular displacement there is an infinitesimal amount of work done given by 𝑑𝑑𝑑𝑑 = 𝜏𝜏𝜏𝜏𝜏𝜏. And since the torque causes rotation in the direction of decreasing 𝜙𝜙, 𝜏𝜏 = −𝑝𝑝𝑝𝑝 sin 𝜙𝜙 • 𝑑𝑑𝑑𝑑 = −𝑝𝑝𝑝𝑝 sin 𝜙𝜙 𝑑𝑑𝑑𝑑, so in rotating the dipole from 𝜙𝜙1 to 𝜙𝜙2 , the work done on the dipole by the torque is: 𝜙𝜙 • 𝑊𝑊 = −𝑝𝑝𝑝𝑝 ∫𝜙𝜙 2 sin 𝜙𝜙 𝑑𝑑𝑑𝑑 = 𝑝𝑝𝑝𝑝 cos 𝜙𝜙2 − 𝑝𝑝𝑝𝑝 cos 𝜙𝜙1 = −Δ𝑈𝑈 = 𝑈𝑈1 − 𝑈𝑈2 1 • This means that the appropriate definition of potential energy for a dipole in an electric field is 𝑈𝑈 = −𝑝𝑝𝑝𝑝 cos 𝜙𝜙 ��⃗, so we can express the • ϕ is still the angle between the vectors �𝒑𝒑⃗ and 𝑬𝑬 ��⃗ �⃗ ⋅ 𝑬𝑬 potential energy as a dot product: 𝑈𝑈 = − 𝒑𝒑 • Recall from mechanics that the equilibria of a system correspond to the extrema of the potential energy function. These are locations where the derivative vanishes and the derivative corresponds to the force (or the torque in the case of rotation), thus these points are where the object is in equilibrium. • Local minima are the points where the equilibria are stable and local maxima are the points where the equilibria are unstable • For the case we are considering (a dipole in an external electric field) there are two values of 𝜙𝜙 where 𝑑𝑑𝑑𝑑 𝑑𝑑𝑑𝑑 ∝ sin 𝜙𝜙 = 0 → 𝜙𝜙 = 0 and 𝜙𝜙 = 𝜋𝜋 • 𝑈𝑈 = −𝑝𝑝𝐸𝐸 where 𝜙𝜙 = 0 and is a minimum – this is the stable equilibrium where the dipole moment is aligned parallel to the electric field • 𝑈𝑈 = +𝑝𝑝𝑝𝑝 where 𝜙𝜙 = 𝜋𝜋 and is a maximum – this is the unstable equilibrium where the dipole moment is aligned antiparallel to the electric field. **SEE EXAMPLE #7**