* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Evidence for specific immune response against P210 BCR
Immune system wikipedia , lookup
DNA vaccination wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Adaptive immune system wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Sjögren syndrome wikipedia , lookup
Molecular mimicry wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Innate immune system wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
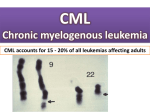
Leukemia (1998) 12, 155–163 1998 Stockton Press All rights reserved 0887-6924/98 $12.00 Evidence for specific immune response against P210 BCR-ABL in long-term remission CML patients treated with interferon T Oka1,2, KJ Sastry3, P Nehete3, SJ Schapiro3, JQ Guo1, M Talpaz4 and RB Arlinghaus1 Departments of 1Molecular Pathology and 4Clinical Immunology and Biological Therapy, The University of Texas, MD Anderson Cancer Center, Houston, TX; and 3Department of Veterinary Sciences, The University of Texas at Science Park, Bastrop, TX, USA Interferon-alpha treatment induces complete cytogenetic remission in 25% of Philadelphia chromosome (Ph)-positive chronic myelogenous leukemia (CML) patients. These remissions are durable unlike remissions induced with other therapies and yet residual leukemia is detectable in most of these patients. Total peripheral blood mononuclear cells (PBMCs) from CML patients in long-term remission following interferon treatment exhibited significantly higher proliferative responses (four- to 15-fold over background) than normals directed against P210 BCR-ABL in extracts of transfected monkey fibroblast cells. Surprisingly, similar enhanced levels of specific proliferative responses were observed with extracts from cells expressing Bcr and/or Abl proteins. In contrast, extracts from vector only or v-Mos-expressing cells had background level responses. Control monkey fibroblast cells lacking BCR-ABL expression failed to induce proliferation over background levels. Normal individuals had no significant responses to Bcr/Abl extracts. On the other hand, peripheral blood mononuclear cells from allogeneic bone marrow transplant CML patients had proliferative responses to cell extracts independent of Bcr-Abl. These data indicate that patients in remission due to alpha-interferon treatment have significantly higher levels of specific cellular immunoreactivity against Bcr/Abl sequences than normal controls, which could play a role in maintaining cytogenetic remission in Ph-positive CML patients. Keywords: BCR-ABL; CML; IFN-␣; cellular immunity Introduction The Philadelphia (Ph) chromosome was initially described as an abnormal chromosome 22 in chronic myelogenous leukemia (CML).1,2 The Ph is the result of a reciprocal translocation of chromosomes 9 and 22, t(9;22)(q34;q11).2 This translocation transposes the c-ABL proto-oncogene from its normal position on chromosome 9 to a new location on chromosome 22 in the central region of the BCR gene. The hybrid BCRABL oncogene produces an abnormal 8.6 kb mRNA that encodes the 210 kDa BCR-ABL protein (P210)3 with an activated tyrosine kinase activity.4,5 In CML, the BCR breakpoint is generally between several small central exons termed exons b2, b3 and b4. These exons correspond to exons 13, 14 and 15 of BCR, respectively. Depending on whether the junction is between either exon b2 or exon b3 of BCR and exon 2 of ABL, two different mRNA are formed with a junction structure known as b2a2 or b3a2, respectively. The resulting P210 BCRABL protein found in CML patients and some acute lymphocytic leukemia (ALL) patients contains either 902 or 927 amino acids encoded by 5⬘ BCR sequences fused to 1096 Correspondence: RB Arlinghaus, Department of Molecular Pathology, Box 89, University of Texas, MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030, USA; 2Current address: Department of Pathology, Okayama University Medical School, Shikata 2-5-1, Okayama 700, Japan Received 7 August 1997; accepted 24 October 1997 amino acids of 3⬘ ABL sequences. Another type of Bcr-Abl protein detected predominantly in Ph-positive ALL contains 426 amino acids encoded by the first exon of BCR fused to the second exon of ABL, termed e1a2, which results in the expression of P185 BCR-ABL.6 CML is a pluripotent stem cell disease that primarily affects myeloid lineages in chronic phase. The disease almost always progresses to the blast crisis stage, which is the terminal stage. Therapy of CML with interferon (IFN) is associated with frequent hematopoietic remissions and complete cytogenetic remissions in about 25% of newly diagnosed treated patients.7 These remissions are rather durable with 92% of patients projected to remain in remission at least 8 years.8 The underlying nature of this remarkable event is not well understood, but two features best characterize it: (1) the durability of these remissions, and (2) the fact that the residual disease can be detected in most of these patients either by studies of patients’ blood and bone marrow, or by detecting BCR-ABL-positive colonies in a clonogenic assay.7,8 Residual disease was detected by the latter assay even in patients who had maintained complete remission for up to 3 years.9 How can we thus explain the presence of residual clonogenic Ph-positive cells, which apparently do not repopulate the bone marrow but maintain a pattern of ‘dormancy’? We propose that complete cytogenetic remissions are the result of an IFN-induced ‘specific’ cellular immune response. Of interest, Chen et al10 reported that immunization of mice with synthetic peptides comprising the b3a2 junction of P210 BCR-ABL primes peptide-specific CD4+, class-II major histocompatibility complex (MHC) restricted T cells. T cell clones specific for the b3a2 peptide were shown to respond in the same way to partially purified P210 BCR-ABL containing the b3a2 junction. These results argue that BCR-ABL junction peptides and/or partially purified P210 BCR-ABL can activate CD4+ helper T cells, and raise the possibility that they may also activate cytotoxic T lymphocyte (CTL) responses in vivo. Of interest, Barrett et al11 demonstrated that normal HLA DR-4 restricted human Tlymphocytes proliferate in response to decapeptides representing both b3a2 and b2a2 fusions of P210 BCR-ABL. In the present study, we demonstrate that peripheral blood mononuclear cells (PBMCs) from CML patients who underwent interferon treatment and are in complete cytogenetic remission are stimulated to proliferate in response to extracts of Bcr-Abl-expressing cells, suggesting a specific cellular immune response against sequences of the oncoprotein P210 BCR-ABL. Materials and methods Cell lines COS-1 cell line (American Type Culture Collection, Rockville, MD, USA) is a simian fibroblast-like cell expressing SV40 Immune response against P210 BCR-ABL T Oka et al 156 large T antigen; it was cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS). Human Bcr-Abl-positive CML cell line K56212 and human Bcr-Abl-negative pre-B leukemia cell line SMS-SB13 were grown in RPMI-1640 with 10% FCS. These cells were cultured at 37°C under a humidified 5.0% CO2 atmosphere. Patient population PBMCs were harvested from heparanized blood of CML patients in complete cytogenetic remission following IFN treatment. All patients completed the necessary consent forms. Clinical details on patients in the study are summarized in Table 1. Cytogenetics A minimum of 20 metaphases in G-banding chromosome studies were examined according to the International System for Human Cytogenetic Nomenclature. Transient transfection and cell extracts COS-1 cell transient transfection was performed by the diethylaminoethyl (DEAE)-Dextran procedure.16 About 1.0 × 108 COS-1 cells were transfected by c-ABL, BCR or BCR-ABL expression plasmids as well as a vector only plasmid as a control; constructs were previously described.17 Cells were harvested 60 h after transfection. Transfections longer than 48 h generate a mixture of both the intact Bcr, Abl or Bcr-Abl proteins, and truncated protein fragments. Cells were resuspended in 25 mM potassium phosphase (pH 7.0) with 2 mM EDTA, 100 mM NaCl, 1 mM PMSF, 50 g/ml of leupeptin, 100 units/ml of aprotinin and 20 mM benzamidine. The cells were homogenized by use of ultrasonic cell disrupter (Virsonic 300; Virtis, NY, USA); the homogenate was centrifuged for 10 min at 3000 g, and supernatant fluid was ultracentrifuged at 110 000 g/min for 90 min at 4°C (Beckman, Fullerton, CA, USA; 50 Ti rotor). Supernatant proteins in cell extracts were adjusted to a concentration that originated from 3.0 × 107 cells/ml (after transfection), aliquoted and stored at −80°C. Cell proliferation assay Peptide synthesis Peptides were made using the Merrifield solid phase method14 either on a modified Vega 250 automatic peptide synthesizer or by the ‘Bag’ method as described by Houghton.15 In either case, removal of T-BOC blocking groups and hydrolysis of the peptide from the resin were accomplished by hydrofluoric acid (HF) treatment at 0°C for 1 h. After HF treatment, either extraction was used to remove various organic compounds and peptides were extracted from the resin with 25% acetic acid. Amino acid sequences of peptides were D60(b3a2)GFKQSSKALQRP; D61(b2a2)-LTINKEEALQRP; D62(e1a2)AFHGDAQALQRPVA and an unrelated Mos peptide (SLCRYLPRELSPSVDSRSC). Peptides were dissolved in PBS at 1.0 mg/ml. Table 1 Patients Clinical data of patients Age Sex Length of time on study (months) RD FJ LV SGd RM CS 42 37 43 31 67 34 M M F M F M 63 46 79 36 110 87 OZ JC BK DP FA PS MM 39 59 57 48 31 35 37 M M M F M F F 57 92 84 57 — — — a Percentage of Ph1(+) cells. 106 IU/day IFN injection. c 106 IU/2 days IFN injection. d CML relapsed patient. b The protocol, based on our previous method18 is shown in Figure 1. About 30 ml of whole heparanized blood was collected by venous puncture from either Ph-positive CML patients undergoing IFN-␣ treatment and in complete cytogenetic remission or healthy volunteers. Peripheral blood mononuclear cells were separated by Ficoll–Hypaque (Histopaque 1077; Sigma, St Louis, MO, USA) centrifugation and were tested for proliferative responses. Three kinds of BCR-ABL junction peptides and unrelated mos synthetic peptide were tested. In addition, several kinds of cell extracts including K562, SMS, and COS-1 cells transfected with BCR, c-ABL or BCR-ABL genes.17 Cells were suspended at 2 × 106 cells/ml of RPMI-1640 supplemented with 10% FCS. A 100 l aliquot of cell suspension was dispensed into each well of a U-bottom 96-well microtiter plate and incubated with differ- Treatment rIFN␣ rIFN␣ rIFN␣ rIFN␣ rIFN␣ rIFN␣ rIF␥ rIFN␣ rIFN␣ rIFN␣ rIFN␣ BMT BMT BMT Initial dose 9.0 MIU q db 10.0 MIU q d 8.0 MIU q d 8.9 MIU q d 6.0 MIU q d 10.0 MIU q d 0.5 mg q d 9.4 MIU q d 10.0 MIU q d 4.4 MIU q d 8.5 MIU q d — — — Last dose 9.2 MIU 4.0 MIU q 8.0 MIU q 8.9 MIU 6.0 MIU q 5.0 MIU q — 9.4 MIU q 4.0 MIU q 4.4 MIU q 6.0 MIU q — — — Duration of response (months) d d odc od d d d d Recent cytogenetics (×103/mm3) WBC Lymph Pha 21 19 48 17 96 43 3.4 10.3 2.6 2.8 3.9 6.2 0.9 1.6 0.9 1.1 0.1 2.0 0.0 0.0 0.0 60.0 5.5 0.0 28 44 57 48 — — — 2.4 6.8 3.0 2.5 4.7 8.5 6.4 0.9 0.3 1.5 1.0 1.6 3.6 3.0 10.0 0.0 5.0 0.0 0.0 0.0 0.0 Immune response against P210 BCR-ABL T Oka et al Western blotting analysis Western blotting analysis was performed according to the technique of Towbin et al.20 Cell extracts were lysed in boiling sodium dodecyl sulfate (SDS) sample buffer for 5 min, separated by 6.5% SDS/PAGE, transferred electrophoretically to polyvinylidene difluoride membrane (Immobilon; Millipore, Bedford, MA, USA) and then treated with 1:20 000 dilution of anti-ABL (8E9) monoclonal antibody.21 Immunoreactive bands were visualized with peroxidase-labeled goat anti-mouse Ig following reaction with the substrate of the enhanced chemiluminescence (ECL) Western blotting system (Amersham, Arlington Heights, IL, USA) and subjected to autoradiography. Results Extracts from Bcr-Abl expressing cells stimulate proliferation of PBMC from CML remission patients Figure 1 Proliferation assay protocol for PBMCs. The procedure is described in Materials and methods. RBCs, red blood cells; PHA, phytohemagglutinin; IL2, interleukin 2. ent concentrations of synthetic peptides, cell extracts or medium alone in a total volume of 200 l. Peptide or extracts were added at 20 l volume. For junction peptides, a 1:10 dilution of peptide stock would yield a concentration in the assay of 10 g/ml. All treatments were done in triplicate wells. The cultures were incubated for 72 h at 37°C in a humidified 5% CO2 atmosphere, and during the last 16–18 h, 1 Ci of 3 H-thymidine (6–7 Ci/m mol; ICN Biochemicals, Costa Mesa, CA, USA) was added. Cells were harvested onto filter strips for estimating 3H-incorporation and counted using a liquid scintillation counter. T cell purification In some experiments, purified T cells were used in the proliferation assay. T cells were purified by the L-leucine methyl ester (Leu-OMe) method as described by Thiele et al19 combined with the IgG conjugate immunobeads method. Briefly after separation by the Ficoll–Hypaque method, PBMCs were resuspended in RPMI 1640, containing 10% FCS and cultured in plastic petri dishes for 1 h at 37°C. Adherent cells bound to the culture matrix were pooled as the macrophage–monocyte fraction. Non-adherent cells were treated with 5 mM Leu-OMe for 50 min in room temperature to lyse residual macrophages and monocytes and washed twice with Dulbecco’s BSS followed by treatment with 10 mg/ml of DNase-I for 30 min at 37°C. After washing twice with PBS, B cells were absorbed with IgG conjugate immunobeads. The eluted fraction was used as the purified T cell fraction. Total PBMCs from CML patients in complete cytogenetic remission as a result of IFN-␣ treatment (Table 1) were tested for proliferative responses against each of three kinds of BCRABL junction peptides: D60 (b3a2); D61 (b2a2); D62 (e1a2) and an unrelated mos peptide. The protocol is described in Figure 1. To our surprise except in a few patients, none of the BCR-ABL junction peptides at any of the three concentrations tested consistently stimulated proliferation at levels higher than PBMCs incubated in medium alone or with a control mos peptide (Figure 2a). However, cell extracts from COS-1 cells transfected with pSG-BCR-ABL plasmid (COS P210) strongly stimulated the proliferation of PBMCs from these CML patients (Figure 2a and b). Cell extracts from K562 cells consistently stimulated proliferation at some dilution conditions but at lower levels than BCR-ABL COS-1 cell extracts (Table 2). Significant proliferation was not observed in several controls including cell extracts of Bcr-Abl negative SMS cells, nontransfected COS-1 cells and COS-1 cells transfected with the vector plasmid pSG5 lacking a gene insert (Figure 2a, Table 2). In addition, PBMCs from healthy volunteers showed no significant response against any Bcr-Abl junction peptide or cell extracts (Figure 3). Table 2 summarizes the proliferative responses of PBMCs from patients and normal controls to the various cell extracts. The stimulation index (SI) is defined as the ratio of average counts per minutes (c.p.m.) of each experimental condition over an average c.p.m. of PBMCs in medium alone. PBMCs from the CML patients showed a three to 11 times higher proliferative response to COS P210 cell extracts compared to COS-1 extract lacking P210. PBMCs from six normal healthy individuals showed no significant response to extracts of COS P210 and that of control COS-1 cells. The SI of PBMCs treated with phytohemagglutinin (PHA) ranged from 17.21 to 206.15 in samples from CML patients and those of healthy volunteers with no significant differences in responses between the two groups. Of interest, patient SG who was relapsing had a very low response to COS P210 (Table 2), suggesting a possible negative correlation of cellular immunity towards Bcr-Abl with leukemia in this patient. Whether these results reflect a role for cellular immunity in the control of the leukemia in the IFNtreated remission patients requires further study. Several experiments were performed to determine whether T cells were responsible for the observed stimulated proliferation. The results indicated that purified T cells alone did not 157 Immune response against P210 BCR-ABL T Oka et al 158 Figure 2 Specific proliferative responses to cell extracts expressing P210 BCR-ABL in PBMCs from Ph-positive CML patients with IFN-␣induced complete cytogenetic remission. (a) PBMCs from CML patient LV with IFN-␣ treatment induced complete cytogenetic remission (see Table 1 for details). PBMCs from patient LV in this experiment responded to PHA (45 623 c.p.m. ± 1378). PBMCs were cultured in the medium alone or with IL-2 (10 g/ml), or in the presence of various peptides or cell extracts. The following abbreviations are shown in the abscissa: BCR3-ABL2 12mer peptide (D60), or BCR2-ABL2 12mer peptide (D61), BCR1-ABL2 12mer peptide (D62), mos peptide, or cell extracts from K562 cells, SMS cells, COS-1 cells transfected with pSGBCR-ABL (COSp210), COS-1 cells transfected with vector plasmid pSG5 (COSpSG5), COS-1 cells transfected with v-mos plasmid (COSv-mos), or COS-1 cells not-transfected (COS). The bar graphs represent results from several dilution series. The cultures were assayed after 72 h incubation using 3H-thymidine (6–7 Ci/mmol) incorporation during the last 16 to 18 h. Bars indicate the means standard deviation (s.d.) of triplicate values of the c.p.m. (b) PBMCs from IFN remission patient DP lack response to vector-only expressing COS-1 cells. In this experiment PBMCs from this patient responded to PHA (3596 c.p.m. ± 1711). Immune response against P210 BCR-ABL T Oka et al Table 2 Patients RD FJ LV SGd RM CS OZ JC BK Nor Nor Nor Nor Nor Nor 1 2 3 4 5 6 159 Proliferative responses of PBMCs from interferon-treated CML patients with BCR-ABL-related proteins Ratioc Stimulation index (SI) COSa+ba COSpSGb COSp210 IL2 K562 SMS COS COSabl COSbcr 4.07 4.84 6.75 2.26 ND 2.52 ND 2.30 ND 1.49 1.30 3.97 1.92 4.46 2.92 1.07 1.46 0.69 0.82 0.84 0.62 0.30 ND 1.55 ND 0.80 ND 0.98 0.68 0.99 0.47 2.05 1.51 1.11 1.16 1.59 ND ND ND ND ND 5.58 7.10 2.78 4.63 ND ND ND ND ND 5.09 7.68 4.40 3.75 ND ND ND ND 1.81 5.31 7.63 3.27 5.48 ND ND ND ND ND ND ND ND ND 6.37 3.66 11.50 1.52 17.22 4.24 9.70 2.94 5.68 6.50 5.38 11.62 3.23 8.40 2.81 8.74 2.53 3.75 4.39 2.67 1.87 2.42 3.64 3.85 0.57 0.56 0.95 0.94 0.86 1.05 0.82 0.79 1.01 0.61 0.86 1.23 1.16 1.16 1.64 0.92 1.32 1.39 ND 1.17 1.08 0.69 1.03 1.58 ND 1.20 1.07 0.57 1.08 1.64 1.43 1.14 0.89 0.80 0.92 1.49 ND 0.88 0.97 0.61 1.20 1.56 1.57 0.53 0.66 0.66 0.82 0.80 1.35 0.47 0.40 0.72 0.62 0.58 SI, average (c.p.m. experimental)/average (c.p.m. cell only). SI (PHA) ranged from 17.21 to 206.15. a COS-1 cells transfected with BCR and c-ABL. b COS-1 cells transfected with vector only (pSG5). c Ratio of SI of COSp210 over COS. d CML relapsed patient. ND, not done. Figure 3 PBMCs from a healthy volunteer did not proliferate in response to COS P210 extracts. PBMCs from this normal donor responded to PHA (38 400 c.p.m. ± 459). D60, D61, D62 represent BCR-ABL peptides as in Figure 2a. D155-P is a phosphotyrosine BCR peptide (VSPSPTTpYRMFRDK); D153 the same BCR peptide made without phosphorylation of the tyrosine residue pSG5; pSG5 is a commercial vector lacking a coding insert; COS v-Mos are transiently transfected COS-1 cells expressing p37v-mos. P210 is the same as COS P210. Immune response against P210 BCR-ABL T Oka et al 160 have increased proliferation in response to COS P210 compared to COS-1 alone but purified T cells mixed with an identical number of irradiated PBMCs from the same patient gave a positive response to COS P210 over and above COS-1 extracts (results not shown). Statistical analysis (Student’s t-test; df, 13) indicated that significantly higher specific proliferative responses to COS P210 were observed for CML patients than for normal subjects (t = 3.2, P ⭐ 0.01). Moreover, proliferative responses generated from K562 cell extracts were significantly higher for CML patients than for normal subjects (t = 2.4, P ⭐ 0.05). In addition, proliferative responses generated from cell extracts of K562 and COS P210 were significantly higher than those from COS cell extracts for patients (t = 2.4, P ⭐ 0.05 and t = 3.7, P ⭐ 0.01, respectively) but not for normal subjects. Extracts of cells expressing Bcr and Abl protein sequences stimulate PBMC proliferation from CML remission patients Because we observed proliferative responses against P210 but not junction BCR-ABL peptides in our assay, we tested whether extracts of cells expressing either Bcr or Abl protein sequences would be recognized by PBMCs from IFN remission patients. Surprisingly, extracts from COS-1 cells transfected with pSGc-ABL, pSGc-BCR or both, stimulated the proliferation of CML patient PBMCs over that of background, but did not stimulate that of healthy volunteers (Table 2). These results indicate that PBMCs from CML remission patients behave as if they have been immunologically primed to recognize normal Bcr and Abl sequences. Student’s t-test (df = 13) indicated that significant differences were observed between patients and normal subjects in their proliferative responses against extracts from COS cells transfected with Abl (t = 4.8, P ⭐ 0.01), Bcr (t = 5.3, P ⭐ 0.001), and a combination of Abl and Bcr (t = 4.2, P ⭐ 0.01). PBMCs from allogeneic transplant CML patients lack a specific response to Bcr-Abl In an effort to determine whether CML patients who had received a bone marrow transplant also possessed the ability to proliferate in response to Bcr-Abl extracts, we assayed PBMCs from three bone marrow transplant patients in remission. PBMCs from allogeneic bone marrow transplanted CML patients showed uniformly high levels of proliferation in response to extracts alone or extracts expressing Bcr, Abl and Bcr-Abl (Table 3). Similar activation was observed with P210 BCR-ABL junction peptides and the mos peptide (data not shown). In these experiments, there was no significant difference between average ratios of SI value obtained with extract of COS-1 cells transfected with P210 BCR-ABL plasmid and that of non-transfected COS-1 cells (Table 3). Samples from CML transplant patients and healthy volunteers had high responses to PHA, indicating that T cells within the PBMC preparation were responsive to antigen stimulation. Immunoblotting analysis of Bcr/Abl expressing cells To establish that COS-1 cells transfected with the pSG5 BCRABL plasmid expressed P210 BCR-ABL, we performed Western blotting analysis using an anti-Abl (8E9) antibody.21 The results showed an intense band of P210 BCR-ABL in transfected but not control COS cell extracts. COS P210 cells contained many degradated forms of the Bcr-Abl protein besides intact P210 BCR-ABL and P145 c-ABL. In contrast, K562 cells had relatively intact P210 BCR-ABL and P145 c-ABL proteins and much lower amounts of degraded BCR-ABL products (Figure 4). Similar levels of degraded Bcr/Abl proteins were observed in COS cells transfected with either BCR or ABL or both cDNAs (not shown). Discussion CML is a pluripotent stem cell disease that primarily affects the myeloid lineage in chronic phase. IFN-␣ induces a complete cytogenetic remission in 25% of treated patients.7 These remissions are durable unlike remissions induced by other therapies and yet residual leukemia is detectable in most of these patients.8 Understanding the mechanisms that govern this remission may help to reveal mechanisms of remission in leukemia in a broader way and help design improved therapies for minimal residual leukemia. However, the nature of this remarkable event is not well understood. Our cell proliferation data are indicative of a statistically significant specific immune response directed against proteins expressed in COS P210 cells; this response was detected in PBMCs from CML patients in complete cytogenetic remission due to interferon treatment. Significant proliferation was also detected with extracts from a CML cell line (K562) expressing P210 BCRABL. However, stimulation of proliferation was much more efficient with COS P210 extracts than with K562 cell extracts. Western blot analysis detected larger amounts of P210 BCRABL degradation products in COS P210 cell extracts, com- Table 3 Proliferative response of PBMCs from allogeneic bone marrow transplanted CML patient’s PBMC to cell extracts expressing BCRABL-related proteins Patients PS MM FA Ratioc Stimulation index (SI) K562 SMS COS COSabl COSbcr COSa+ba COSpSGb COSp210 1.14 2.02 2.45 — — 6.58 4.75 2.96 7.00 3.86 6.17 6.26 4.23 4.03 6.82 4.39 4.66 6.62 — — 2.80 3.19 4.80 7.84 SI, average (c.p.m. experimental)/average (c.p.m. cell only). SI (PHA) ranged from 50.52 to 111.54. a COS-1 cells transfected with BCR and c-ABL. b COS-1 cells transfected with vector only (pSG5). c Ratio of SI of COSp210 over COS. Normal healthy controls are shown in Table 2. 0.67 2.38 1.12 Immune response against P210 BCR-ABL T Oka et al Figure 4 Western blot analysis of P210 BCR-ABL expressed in K562 cells, COS-1 cells transfected with pSG-BCR-ABL and control COS-1 cells. The expression of P210 BCR-ABL was detected by antiAbl 8E9 monoclonal antibody. An equal amount of protein was applied to each gel lane. pared to that in K562 cells (Figure 4). This may suggest that higher expression of Bcr-Abl degradation products present in the transfected COS-1 cell extracts compared to K562 cell extracts might contribute to the higher efficiency of presentation of Bcr-Abl peptides by antigen-presenting cells contained within the PBMC preparation. The specificity of these phenomena observed in PBMCs of IFN-treated CML patients strongly suggests that IFN treatment stimulates cell-mediated immunity against BCR-ABL expressing leukemic cells within CML patients. Because of the complexity of these assays and the reagents used, we wanted to test the reproducibility of these findings by another group of individuals. Dr Talpaz’s immunology group were able to confirm these findings but used 5–7-day incubation periods with PBMCs instead of 3 days as in this study (X Qui, C Newell, K Cheng and M Talpaz, unpublished results). Surprisingly, extracts from COS-1 cells expressing normal Bcr and Abl proteins or both also induced significant proliferation of PBMCs from CML long-term remission patients. In contrast, vector only transfected cells and mos-transfected lacked stimulatory activity. These findings suggest that Bcr and Abl protein sequences are recognized by memory T cells as a result of IFN treatment. We cannot eliminate the possibility that the cell-mediated immune responses are generated against proteins induced in COS-1 cells by P210 BCR-ABL. This possibility is in our view unlikely because Bcr and Abl overexpression would have to induce the same or related antigens. Also, these experiments were not done with stably transfected cells but with COS-1 cells transiently transfected for only 3 days in which less than 10% of the cells are expressing either Bcr-Abl or Bcr/Abl proteins. Before beginning these studies, we expected that BCR-ABL junction peptides would stimulate proliferation of PBMCs from IFN responders. However, such junction peptides consistently failed to activate PBMCs from IFN responders. These findings suggest that the response to COS P210 extracts is due to sequences within Bcr or Abl proteins, but not to junction sequences. In this regard we observed that extracts of COS-1 cells expressing Bcr, Abl or both Bcr and Abl proteins induced significant proliferation of PBMCs from IFN responders. The response was similar in magnitude to that observed with COS P210 extracts. These results were puzzling to us at first. However, these findings could be explained by previous observations made by others in which normal proteins such as HER2/neu expressed in high amounts appeared to be sufficient to activate T cells.22 A mechanism for recognition of normal antigens has been proposed by Ioannides et al23 and later extended by Fisk et al.22 Their results showed that a 9mer peptide from HER-2 (369–377) was efficient in sensitizing human CEM cells for lysis by CTL lines that were ovarian tumor-specific. Disis et al24 demonstrated induction of CTLs from normal lymphocytes with peptides derived from the HER-2/neu proto-oncogene. These results suggest that over expression of a normal antigen could in some situations impart immunogenicity to the protein and lead to a leukemic cell-specific CTL response. Therefore in the case of CML, IFN treatment may enhance the cell-mediated immune response system in a way that allows efficient induction and expression of helper and cytotoxic T lymphocytes directed against BcrAbl-expressing cells. Possibly high expression of the Bcr-Abl protein antigen leads to high amounts of presentation by class I and II molecules in the presence of IFN, thereby overcoming the natural lack of T cell activation by normal gene products. That this interpretation may have some merit is supported by our observation that extracts of COS-1 cells overexpressing Bcr or Abl proteins provide high levels of protein fragments (Figure 4) that could be presented by antigen-presenting cells within the PBMC preparation, leading to T cell activation. Thus, either Bcr protein fragments or Abl protein fragments would trigger memory T cells that were initially primed by leukemic cells expressing Bcr-Abl proteins, but COS-1 cells not expressing high amounts of Bcr or Abl proteins would not yield extracts that stimulated PBMC proliferation. Of interest in this regard, SMS cells are a human leukemia cell line expressing moderate levels of Bcr and Abl proteins yet extracts from these cells did not stimulate proliferation of PBMCs from IFN-treated patients (Table 2). This result reinforces the interpretation that it is overexpression combined with protein degradation of Bcr and Abl sequences that lead to activation of patient PBMCs. In contrast to the proliferative response exhibited by the PBMCs from IFN responders, PBMCs from allogeneic bone marrow transplantation patients were reactive to every antigen tested including zenotypic stimulation, suggesting that the immune reactivity in bone marrow transplant patients is an allo-antigen-specific mixed lymphocyte reaction, which is distinct from that mediated by Bcr/Abl sequences. 161 Immune response against P210 BCR-ABL T Oka et al 162 Bcr-Abl junction peptides have been shown to elicit a CD4 response in a healthy, normal individual.25 In these studies a 17 amino acid b3 a2 junction peptide was used to generate a Bcr-Abl-specific T cell line from a healthy donor that proliferated in response to the peptide. Proliferative responses have also been observed in normal donor cells (three of seven) tested against a 25 amino acid b3 a2 peptide.26 In this report, four of four HLA-A3 donor cell populations also produced cytotoxic T lymphocytes (CTLs) in vitro in response to a junction peptide mixture. These CTLs lysed an allogeneic HLAA3-matched leukemia cell line pulsed with the same peptide mixture. The best inducer was a KQSSKALQR (b3 a2 peptide)/ATGFKQSSK (b3 peptide) mixture. We cannot explain why the long-term remission patients tested in our study did not consistently respond to Bcr-Abl junction peptides. The amount of peptide used in our study could be a factor as the concentration of junction peptide used in one study26 (50 g/ml) was five times higher than the concentration tested in our study (Figure 2a). Also, longer exposures to PBMCs (we used 3 days) from IFN remission patients might be a factor. Nevertheless, our results suggest that IFN may enhance presentation of normal antigens in general as suggested by the relative high frequency of autoimmune symptoms seen in some of these patients (Talpaz, unpublished results). Thus, these patients may generate an antileukemic response due to enhanced recognition of Bcr and Abl gene products. Our data raise questions about whether or not CML patients with active disease are able to mount an immune response against BCR-ABL expressing leukemic cells. One possible immune deficit is the lack of appropriate helper T cell function necessary to elicit an effective immune response. Barrett et al11 showed that normal individuals with DR4 class II haplotype had T cells that proliferated in response to the two forms of Bcr-Abl junctions: b2a2 and b3a2. However, individuals with DR 1,2,3 and 5 did not. Possibly, IFN treatment may activate a T cell immune response in those patients that have the DR4 haplotype. Of interest, only about 25% of CML patients respond positively to IFN treatment. It will be of interest to determine whether IFN-sensitive patients share the same HLA pattern. Another possibility is that BCR-ABL expression down-regulates class I and/or class II MHC gene expression, but in some patients, IFN treatment is able to overcome this down-regulation. IFN might function in another way by overcoming T cell anergy against Bcr-Abl antigen presentation. In this case, IFN would activate cell-mediated immune responses against any cell that overexpresses Bcr-Abl proteins. Other possibilities include lack of appropriate HLA expression in CML patients and IFN treatment would activate the pertinent HLA allele, or BCR-ABL expression down-regulates cytotoxic T cell function and IFN stimulates CTL function. The latter possibility seems unlikely, as patients in chronic phase are typically free of immune suppression symptoms. This research has important implications with regard to possible immunotherapy of CML. If immunologic peptides derived from Bcr or Abl proteins specific to CML could be identified, those peptides could form the basis of a future immunotherapeutic vaccine. Fragmented P210 BCR-ABL could also be used for this purpose. In addition, if CTL effector clones can be isolated that are specific for P210 BCR-ABL expression in HLA-matched cell lines and/or Bcr/Abl peptidetreated cells from the same patient, these clones would be useful for in vitro purging of marrow cells from CML patients and may also be useful for treating allogeneic bone marrow transplant patients. Further investigations are underway to expand this study and to examine basic immunologic parameters. Acknowledgements This work was supported by NIH grants CA65611 and CA16672 and Hubert L Stringer Chair. We would like to gratefully acknowledge the technical assistance of Drs Dai Lu and Jiaxin Liu as well as the assistance of Tammy Trlicek for manuscript preparation. We also thank Dr Brad McIntyre for his help in preparing purified T cells and monocytes. References 1 Nowell PC, Hungerford DA. A minute chromosome in human chronic granulocytic leukemia. Science 1960; 132: 1497–1499. 2 Rowley JD. A new consistent chromosomal abnormality in chronic myelogenous leukemia identified by quinacrine fluorescence and Giemsa staining. Nature 1973; 243: 290–293. 3 Shtivelman E, Lifschitz B, Gale RP, Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukemia. Nature 1985; 315: 550–554. 4 Konopka JB, Watanabe SM, Witte ON. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell 1984; 37: 1035–1042. 5 Kloetzer W, Kurzrock R, Smith L, Talpaz M, Spiller M, Gutterman J, Arlinghaus R. The human cellular abl gene product in the chronic myelogenous leukemia cell line K562 has an associated tyrosine protein kinase activity. Virology 1985; 110: 230–238. 6 Hermans AS, Heisterkamp N, von Lindern M, vanBaal S, Meijer D, van der Plas D, Wiedemann LM, Groffen J, Bootsma D, Grosveld G. Unique fusion of bcr and c-abl genes in Philadelphia chromosome positive acute lymphoblastic leukemia. Cell 1987; 51: 33–40. 7 Kantarjian HM, Deisseroth AB, Kurzrock R, Estrov Z, Talpaz M. Chronic myelogenous leukemia; a concise update. Blood 1993; 82: 691–703. 8 Dhingra K, Kurzrock R, Baine R, Eastman S, Kantarjian H, Gutterman JU, Talpaz M. Minimal residual disease in interferontreated chronic myelogenous leukemia; results and pitfalls of analysis based on polymerase chain reaction. Leukemia 1992; 6: 754–757. 9 Talpaz M, Estrov Z, Kantarjian H, Ku S, Foteh A, Kurzrock R. Persistence of dormant leukemic progenitors during interferoninduced remission in chronic myelogenous leukemia. J Clin Invest 1994; 94: 1383–1389. 10 Chen W, Peace DJ, Rovira D. T-cell immunity to the joining region of P210 BCR-ABL protein. Proc Natl Acad Sci USA 1992; 89: 1468–1472. 11 Barrett AJ, Jiang YZ, Kars A, Gordon AA, Datta A. HLA DR-4 restricted T-lymphocytes recognize decapeptides representing the novel fusion region of the BCR-ABL protein in CML. J Cell Biochem 1992; 16D (Suppl.): 26 (Abstr.). 12 Lozzio CB, Lozzio BB. Human chronic myelogenous leukemia cell line with positive Philadelphia chromosome. Blood 1975; 45: 321–334. 13 Smith RG, Dev VG, Shannon WA Jr. Characterization of a novel pre-B leukemia cell line. J Immunol 1981; 126: 596–602. 14 Merrifield RB. Solid phase peptide synthesis. J Am Chem Soc 1963; 85: 2149–2154. 15 Houghten RA. General method for the rapid solid-phase synthesis of large numbers of peptides; specificity of antigen–antibody interaction at the level of individual amino acids. Proc Natl Acad Sci USA 1985; 76: 4350–4354. 16 Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, 1989, p 16.42. 17 Lu D, Liu J, Campbell M, Guo JQ, Heisterkamp N, Groffen J, Canaani E, Arlinghaus RB. Tyrosine phosphorylation of p160BCR by P210 BCR-ABL. Blood 1993; 82: 1257–1263. 18 Nehete PN, Johnson PC, Shapiro SJ, Arlinghaus RB, Sastry KJ. Immune response against P210 BCR-ABL T Oka et al 19 20 21 22 Cross reactive T-cell proliferative responses to V3 peptides corresponding to different geological HIV-1 isolates in HIV-seropositive individuals. J Clin Immunol 1996; 16: 115–124. Thiele DL, Kurosaka M, Lipsky PE. Phenotype of the accessory cell necessary for mitogen-stimulated T and B cell responses in human peripheral blood; delineation by its sensitivity to the lysosomotropic agent, L-leucine methyl ester. J Immunol 1993; 131: 2282–2290. Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 1979; 76: 4350–4354. Guo JQ, Lian JY, Xian YM, Lee M-S, Deisseroth AB, Stass SA, Champlin RE, Talpaz M, Wang JYJ, Arlinghaus RB. BCR-ABL protein expression in peripheral blood cells of chronic myelogenous leukemia patients undergoing therapy. Blood 1994; 83: 3629– 3637. Fisk B, Blevins TL, Wharton JT, Ioannides CG. Identification of an 23 24 25 26 immunodominant peptide of HER-2/neu proto-oncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J Exp Med 1995; 181: 2109–2117. Ioannides CG, Ioannides MG, O’Brian CA. T cell recognition of oncogene products: a new strategy for immunotherapy. Mol Carcinogen 1992; 6: 77–82. Disis ML, Smith JW, Murphy AE, Chen W, Cheever MA. In vitro generation of human cytolytic T cells specific for peptides derived from the HER-2/neu proto-oncogene protein. Cancer Res 1994; 54: 1071–1076. ten Bosch GJA, Joosten AM, Kessler JH, Melief CJM, Leeksma OC. Recognition of peptides corresponding to the joining region of p210BCR-ABL protein by human T cells. Leukemia 1995; 9: 1344–1348. Bocchia M, Korontsvit T, Xu Q, Mackinnon S, Yang SY, Sette A, Scheinberg DA. Specific human cellular immunity to bcr-abl oncogene-derived peptides. Blood 1996; 87: 3587–3592. 163