* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Core Clinical Problem 52: Murmur Summary ΔΔ (Index Conditions
Cardiovascular disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Turner syndrome wikipedia , lookup
Echocardiography wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Heart failure wikipedia , lookup
Electrocardiography wikipedia , lookup
Marfan syndrome wikipedia , lookup
Coronary artery disease wikipedia , lookup
Rheumatic fever wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Artificial heart valve wikipedia , lookup
Myocardial infarction wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Cardiac surgery wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Aortic stenosis wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Atrial septal defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
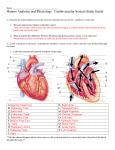
Core Clinical Problem 52: Murmur Summary ΔΔ (Index Conditions numbered) Vascular / haematological Infective Traumatic Autoimmune Metabolic Inflammatory Neoplastic Benign Congenital/ hereditary Degenerative Aortic dissection (6) Atrial ball-valve thrombi Rheumatic fever (1b) Endocarditis (1c) Aortic dissection (6) Cardiomyopathies (5) Cardiomyopathies (5) Atrial tumours Innocent murmurs (3) Congenital heart disease (2) Septal defects Tetralogy of Fallot Patent ductus arteriosis Coarctation of the aorta Marfan’s syndrome (4) Hypertrophic cardiomyopathy (5) Valvular heart disease (1a) Stenotic Regurgitative Basic Sciences Structure of the heart 1 Development of the heart Cell layer of origin = Mesoderm Left-right positioning determined by factor called nodal, wafted left by cilia 1. Formation of primitive heart tube from right and left endocardial heart tubes. 2. Cardiac looping- the heart tube bends and grows into the pericardial cavity. Dilations form: bulbous cordis → ventricle → atrium → Right and left horns of sinus venosum http://www.youtube.com/watch?v=g5UTzj9LOhQ 3. Cardiac septation Ventral and dorsal endocardial cushions → Septum intermedium Septum primum and septum secondum grown down from root of atria leaving foramen ovale Muscular ventricular septum grows up from floor of ventricle Bulbar ridges in bulbus cordis → Spiral aortico-pulmonary septum → Fuses with septum intermedium and then with ventricular septum A good video of the whole process: http://www.youtube.com/watch?v=5DIUk9IXUaI DIASTOLE Isovolumic reslaxation End systolic volume (approx. 50%) LV pressure < LA pressure (venous Brief rise in aortic pressure return) Aortic valve closes Mitral valve opens LV pressure < Aortic pressure Rapid filling (+ active relaxation Ejection i.e. sucking in) The Aortic valve opens LV pressure = LA pressure cardiac LV pressure > Aortic pressure Filling temporarily stops cycle Isovolumic contraction (diastasis) LV pressure < Aortic pressure LA contracts Mitral valve closes LV pressure < LA pressure LV pressure > LA pressure Further filling (15-20% Ventricles begin to contract contribution, at rest) SYSTOLE End diastolic volume 2 1. Valvular Heart Disease a. Degenerative Valve Disease Pathophysiology Leads to heart failure, which is often responsible for signs and symptoms... Mitral Stenosis → o Left atrial hypertrophy + dilatation o Pulmonary hypertension o Right ventricular hypertrophy, dilatation + failure. Mitral Regurgitation → o Pulmonary hypertension if acute o Left ventricular enlargement to maintain S.V. Aortic Regurgitation → Left ventricular enlargement + failure Symptoms ...Of induced heart failure: Left sided- dyspnoea, orthopnoea Right sided- abdominal pain and distension, lethargy Aortic stenosis: Exercise induced syncope, angina, dyspnoea PMH- Rheumatic fever Signs ...Of induced heart failure Murmur Aortic Stenosis Aortic Regurgitation Mitral Stenosis Mitral Regurgitation Tricuspid Regurgitation Location of lesion Semi-lunar Valves AV Valves Timing Systolic Stenosis (Ejection) Regurgitation (Pan) Diastolic Regurgitation (Early) Stenosis (Mid) Radiates to carotids Slow rising carotid pulse Heard at tricuspid area, patient lent forward, held expiration Bounding/Collapsing pulse Heard best with patient rolled 450 to left Malar flush Low volume pulse Radiates to left axilla Pulsatile, enlarged liver Investigations CXR: enlargement of chamber behind problem ECG: changes reflecting hypertrophy of effected chamber Echocardiography: shows volume and flow changes Management Surgery: Balloon valvotomy/ replacement For HF eg. Diuretics 3 Prophylactic antiobiotics against infective endocarditis are not recommended, unless the patient is undergoing a GI or GU procedure at a site of suspected infection. b. Rheumatic Fever Pathophysiology Abnormal immune response to infection with Group A β-haemolytic strep Now rare in developed countries (sanitation, antibiotics, reduced virulence) Features Preceding pharyngitis 2-6 weeks before Diagnostic criteria: 2 major / 1 major + 2 minor + evidence of previous strep (culture or ASOT) Major: 1. Polyarthritis- ankles, knees + wrists, tender, moderate redness + swelling, flitting between joints for 1-2 months. 2. Pancarditis Endocarditis- significant murmur, valve dysfunction Myocarditis- heart failure Pericarditis- pericardial friction rub, effusion, tamponade 3. Erythema marginatum- pink macular rash spreading outwards 4. Sydenham’s chorea- after 2-6 months, involuntary movements + emotionally labile for 3-6 months 5. Subcutaneous nodules- rare, extensor surfaces, hard, painless, pea-sized Minor: o Fever o Polyarthralgia o Raised ESR, CRP, leucocytosis o Prolonged P-R interval on ECG o Previous history of rheumatic fever Management Acute- high dose aspirin (corticosteroids if ineffective), pericardiocentesis if required, treat heart failure with ACE inhibitors + diuretics Prophylaxis (recurrence prevention)- monthly benzathine penicillin IM Complications Scarring + fibrosis of heart valves → Mitral stenosis (commonest) 4 c. Infective endocarditis Risk factors Congenital heart disease (except secundum ASD) Prosthetic valves Causes of bacteraemia: IVDU Dentistry UTI, catheters Surgery/ TOP/ fracture Most caused by α-haemolytic streptococcus e.g. viridans Presentation Fever + New murmur = IE until proven otherwise Rigors Night sweats Weight loss Pallor Splinter haemorrhages Necrotic skin lesions o Osler’s: tender, finger pulp o Janeway: non-tender, palms Retinal infarcts (Roth spots) Clubbing (late) Splenomegaly Arthritis/arthralgia Neurological signs from cerebral infarction Investigations FBC: normochromic normocytic anaemia, ↑ neutrophils Urine: microscopic haematuria ESR ↑ Blood cultures (do multiple before starting antibiotics) Echocardiography: may detect vegetation (mass of bacteria, neutrophils and fibrin) Management Antibiotics e.g. High dose penicillin + an aminoglycoside (gentamicin) IV for 6 weeks Surgical removal of any prosthetic material may be necessary Complications Glomerulonephritis → acute renal failure Emboli → Abscess in brain/kidney/spleen/GI/ lung (if right-sided valve) 5 2. Congenital Heart Disease Cyanotic and non-cyanotic congenital heart disease Acyanotic congenital heart disease Left to right shunts o Ventricular septal defect o Persistent ductus arteriosus o Atrial septal defect Outflow obstruction o Pulmonary stenosis o Aortic stenosis o Coarctation of the aorta Cyanotic congenital heart disease Tetralogy of Fallot Transposition of the great arteries Complete atrioventricular septal defect Atrial septal defect Pathophysiology Secondum ASD involving centre of atrial septum and foramen ovale Primum ASD: partial atrioventricular defect. The communication is between the bottom end of the atrial septum and the AV valve, with a three-leaflet regurgitant left AV valve Presentation Commonly asymptomatic Recurrent chest infections/ Wheeze Symptoms of heart failure On auscultation o Ejection systolic murmur heard best at upper left sternal edge (due to increased outflow from left-to-right shunt) o Fixed widely split second heart sound o In AVSD- pansystolic apical murmur (AV valve regurgitation) Investigations CXR: Cardiomegaly, enlarged pulmonary arteries, increased pulmonary vascular markings. ECG: Secondum: Partial right bundle branch block, right axis deviation Partial AVSD: Left ‘superior QRS’ axis deviation Echocardiogram (cross-sectional): Mainstay of diagnosis Management Correction of secondum ASD: cardiac catheterisation + occlusive device Correction of partial AVSD: surgical Should take place at 3-5 years Complications Right heart failure Arrhythmias in adulthood 6 Ventricular septal defect Pathophysiology Perimembranous- located adjacent to the tricuspid valve Muscular- surrounded by muscular septum Small = smaller than aortic valve (up to 3 mm), Large if same size of bigger Presentation Small VSD Asymptomatic Thrill (lower sternal edge) Loud pansystolic murmur (lower left sternal edge) Quiet pulmonary second sound Large VSD Heart failure, with breathlessness and failure to thrive after 1 week, tachypnoea, tachycardia and hepatomegaly. Recurrent chest infections Soft pansystolic murmur/ no murmur Apical mid-diastolic murmur (increased flow through mitral) Loud pulmonary second sound (raised pulmonary artery diastolic pressure) Investigations Chest x-ray: normal in small; large shows cardiomegaly, enlarged pulmonary arteries, increased pulmonary vascular markings, and pulmonary oedema ECG: normal in small; large shows biventricular hypertrophy and pulmonary hypertension (T wave upright, if inversted suggests no PH) Echocardiogram: assess anatomy and haemodynamic effects with doppler Management Small lesions mostly close spontaneously. Promote good dental hygiene; prophylactic ABx no longer recommended Large lesions also require: Additional calorie input Diuretics and captopril for HF Surgery at 3-6 months Complications Pulmonary vascular disease (capillaries damaged by high pressures) Eisenmenger’s (unusual before second decade of life) Bacterial endocarditis Patent ductus arteriosus Pathophysiology Failure to close by 1 month post-term (not pathological in preterm infants) Flow occurs from aorta to pulmonary artery due to reversal of pressure gradient (i.e. left-toright shunt). Increased risk in preterm or unwell babies (sepsis, pulmonary hypertension…) 7 Presentation Continuous murmur beneath left clavicle Collapsing/ bounding pulse (high pulse pressure) Rarely symptomatic Investigations Chest x-ray: usually normal, if large shows same features as large VSD ECG: usually normal, if large shows same features as large VSD Echocardiogram- cross-sectional with doppler Management Prostaglandin inhibitor: indomethacin Closure at 1 yr by cardiac catheterisation and insertion of coil/ occlusive device Complications Rarely, right heart failure, pulmonary hypertension Bacterial endocarditis Aortic stenosis Pathophysiology Aortic valve leaflets partially fused together restricting outflow Often associated with mitral stenosis and coarctation of the aorta Presentation Asymptomatic murmur Small volume, slow rising pulse Carotid thrill Ejection systolic murmur- maximal upper right sternal edge, radiates to neck Delayed soft aortic second sound Apical ejection click Severe neonatal presentation: o Heart failure o Duct-dependent systemic circulation → Shock If severe: o Reduced exercise tolerance (dyspnoea) o Chest pain on exercise o Exercise syncope Investigations CXR: Normal/ Prominent left ventricle with post-stenotic dilatation of ascending aorta ECG: May show left ventricular hypertrophy (deep S in V2, tall R in V6), of left ventricular strain if severe (inverted T wave in V6) Echocardiography Management Indications for intervention: symptoms on exercise or high resting pressure gradient on echo (> 64 mmHg across aortic valve) → Balloon valvotomy 8 Most with early severe disease will eventually require valve replacement Pulmonary stenosis Pathophysiology Pulmonary valve leaflets partially fused restricting outflow from right heart Presentation Asymptomatic Ejection systolic murmur best at upper left sternal edge +/- thrill Ejection click at upper left sternal edge Soft or absent second pulmonary sound If severe o Neonate with duct-dependent circulation o Prolonged right ventricular impulse o Delayed pulmonary valve closure on auscultation Investigations Chest x-ray: Normal/ Post-stenotic dilation of pulmonary artery ECG: Evidence of right ventricular hypertrophy (Upright T wave in V1) Echocardiography Management Indications for intervention: symptoms on exercise or high resting pressure gradient on echo (> 64 mmHg across pulmonary valve) → Balloon valvotomy Complications Right heart failure Coarctation of the aorta Pathophysiology Constriction of aorta in area where ductus arteriosus joins Condition worsens gradually over years Presentation Asymptomatic Systemic hypertension in right arm (always) Ejection systolic murmur between shoulder blades Collaterals Radio-femoral delay Investigations CXR: Rib notching (large collateral intercostal arteries), visible notch in descending aorta ECG: Left ventricular hypertrophy (deep S in V2 + tall R in V6), left ventricular strain if severe coarctation/hypertension (T wave inversion in V6) MR angiography Echocardiography 9 Management When severe, correction by cardiac catheterisation and stenting Left heart outflow obstruction in a sick infant → Duct-dependent lesions Features Interruption of the aortic arch Murmur, heart failure, At site of DA, + VSD circulatory collapse, Associated with DiGeorge syndrome weak femorals, BP in arms > in legs Hypoplastic left heart syndrome Circulatory collapse, all Underdevelopment of entire left heart peripheral pulses Mitral + aortic atresia, coarctation, ASD weak/absent Management ABC Immediate prostaglandin infusion ABC Immediate prostaglandin infusion Surgery (complex) Tetralogy of Fallot Pathophysiology 1. Large VSD 2. Overriding aorta 3. Right ventricular outflow obstruction (subpulmonary stenosis) 4. Right ventricular hypertrophy Presentation Antenatal diagnosis Murmur in first 2 months of life Cyanosis if severe Collapse (can be duct dependent) Hypercyanotic spells- rapid onset cyanosis, with irritability/ inconsolable crying, breathlessness, pallor Clubbing (older children) Loud, harsh ejection systolic murmur at left sternal edge Single second heart sound Investigations Chest x-ray: Normal/ In older children- small heart with uplifted apex, pulmonary artery ‘bay’ (dip), decreased pulmonary vascular markings ECG: Normal at birth, right ventricular hypertrophy when older (Upright T in V1 with ‘pure R’ i.e. no S wave) Echocardiography, though cardiac catheterisation may also be necessary Management Cyanosed neonate → shunt inserted between subclavian artery and pulmonary artery (Blalock-Taussig shunt), or balloon dilatation of pulmonary outflow tract Surgery (6 months): Closure of VSD and relieve right outflow obstruction 10 Hypercyanotic spells lasting > 15 mins → Sedation and morphine IV propranolol IV fluids Bicarbinate (correct acidosis) Muscle paralysis and ventilation (reduce oxygen demand) Complications Hypercyanotic spells → MI, stroke, death Transposition of the great arteries Pathophysiology Aorta connected to right ventricle and pulmonary artery to left ventricle (deoxygenated blood returned to body and oxygenated returned to lungs), Co-existing VSD, ASD, or PDA associated and vital to be compatible with life. Presentation Duct dependent so present at day 1-2 when closure occurs Cyanosiso Profound and life-threatening o Milder with other abnormalities causing blood mixing. Clubbing if present after 1 year (rare) Single second heart sound, and signs of associated abnormalities Investigations Chest x-ray: Narrow upper mediastinum, ‘egg on side’ shape (RV hypertrophy and anteriorposterior aligned vessels), increased pulmonary vascular markings ECG: Normal Echocardiography Management Cyanotic neonate → Prostaglandin infusion (maintain DA), balloon atrial septostomy Surgery: Open surgical correction in the first few days of life Complete atrioventricular septal defect Pathophysiology Mostly children with Down’s syndrome Defect in the middle of the heart Single regurgitant five-leaflet valve stretching across entire heart Pulmonary hypertension Presentation Detection at antenatal screening Cyanosis at birth or heart failure in first 2-3 weeks Echocardiography screening of baby with Down’s syndrome 11 Investigations ECG: Superior axis Echocardiography Management Medical management of heart failure and surgical correction at 3-6 months Tricuspid atresia Pathophysiology Only left ventricle functional, right small. Blood circulated through foramen ovale from RA to LA then from LV through a VSD to right outflow tract. Presentation: Cyanosis- new-born or duct dependent Management Shunt between subclavian artery and pulmonary artery (Blalock-Taussig) Pulmonary artery banding to reduce pulmonary blood flow if breathless Surgical: correction not possible, instead superior and inferior vena cava connected to pulmonary artery. 3. Innocent murmurs Commonly heard in childhood or during pregnancy Ejection systolic o Caused by turbulent flow o Soft, heard at left sternal edge (right heart) o Or short and ‘buzzing’ over apex (aorta) o No thrill, no radiation o Asymptomatic o Often heard in anaemia or fever (increased cardiac output) Venous hum o Turbulent flow in head and neck veins o Continuous rumble under either clavicle o Distinguished from PDA because it disappears on lying down o Mammary souffle = venous hum from engorged breast vessels in pregnancy 12 4. Marfan’s Syndrome Pathophysiology Autosomal dominant mutation in fibrillin-1 gene Connective tissue disorder: decreased extracellular microfibril formation. Features Major (>2 to diagnose): Lens dislocation, usually upwards Aortic dissection/ dilatation (→ aortic regurgitation) Dural ectasia (large neural canal) Arachnodactyly (long fingers) Arm span > height Pectus deformity (carinatum or excavatum) Scoliosis Pes planus (flat feet) Minor: Mitral valve prolapse High-arched palate Joint hypermobility Kyphoscoliosis Hernias (diaphragmatic/inguinal) Investigations MRI (aid diagnosis by showing dural ectasia) Echocardiogram (annual) Management β-blocker helps prevent cardiovascular complications Prophylactic surgery to prevent aortic dissection when diameter >5cm Consider inducing precocious puberty in very tall girls 5. Cardiomyopathies Cardiomyopathy = Primary myopathy of cardiac muscle Types Dilated Hypertrophic Restrictive Presentation: usually with heart failure, as systolic or diastolic dysfunction, or both. Other presentations include chest pain, syncope and sudden death Investigation: Echocardiography, ECG, Chest x-ray 13 Causes Dilated Diffuse coronary artery disease Infection Viral e.g. coxsackie Chagas disease HIV Toxoplasma Toxins Alcohol Chemo e.g. doxorubicin Granulomatous e.g. sarcoid Metabolic e.g. thyrotoxicosis Familial tendency with variable inheritance Pathophysiology Features Systolic dysfunction ECG Non-specific ST and T changes Q waves +/- bundle branch block For heart failure… Diuretics, ACE inhibitors, Angiotension receptor blockers, Beta blockers, Digoxin Pacing: ICD or biventricular Anticoagulationprophylactic for mural thrombi should be considered Transplant 20% mortality first year, 10% per year after Treatment Prognosis LV and RV failure Cardiomegaly Functional AV valve regurgitation S3 S4 Hypertrophic Genetic (most cases): numerous mutations identified, most autosomal dominant but many cases are spontaneous Diastolic dysfunction +/- outflow obstruction Starting at 20-40 years: Exertional dyspnoea, angina, syncope Sudden death Ejection murmur +/- mitral regurge S4 Bifid carotid pulse No cardiomegaly LV hypertrophy and ischaemia Deep septal Q waves Beta blockers +/- verapamil (rate limiting and –ve inotrope) +/- disopyramide +/- septal myotomy +/- alcohol ablation AV pacing Transplant Restrictive Non-obliterative Myocardial infiltration by abnormal substance Genetic Haemochromatosis Lysosomal storage diseases (Fabry’s, Gauchers) Connective tissue Amyloidosis Diffuse systemic sclerosis Other Hypereosinophilia Sarcoid Radiation Obliterative Fibrosis of endocardium + subendocardium Diastolic dysfunction Exertional dyspnoea and fatigue LV +/- RV failure Functional AV valve regurgitation Mild or no cardiomegaly LV hypertrophy OR Low voltage Endocardial resection Phlebotomy for haemochromatosis Hydroxyurea for hypereosinophilia Not transplant- would generally recur 1% annual risk of sudden death 70% 5 year mortality 14 6. Aortic Dissection Pathophys. Wall damaged → Blood enters between intima and media in false channel, obstructing flow through normal lumen Life-threatening complications: (most likely in acute dissections) Compromise of blood supply to tributary arteries including coronary arteries Aortic valve dilatation and regurgitation Heart failure Fatal aortic rupture into pericardium of left pleural space Underlying causes: Hypertension Atherosclerosis Acquired connective tissue disorder: Behçet's syndrome, Giant cell arteritis, Takayasu’s arteritis Congenital connective tissue disorder: o Marfan’s syndrome o Ehler’s Danlos syndrome o Turner’s syndrome o Coarctation of the aorta o Bicuspid aortic valve o Familial thoracic aortic aneurysm Trauma: deceleration injury Iatrogenic: valve surgery, aortic catheterisation Types: Type I: Starts in ascending aorta and extends to or beyond arch. 50% Type II: Start in and confined to ascending aorta. 35% Type III: Start in descending aorta and usually extend distally. 15% Symptoms and Signs Sudden tearing pain in central chest or intrascapular May radiate to back Syncope Branch occlusions→ Hemiplegia (carotid) Unequal arm pulses/BP/Acute arm ischaemia Paraplegia (anterior spinal) Anuria (renal) Investigation: Chest x-ray, transoesophageal echo/ CT angiogram/ MR angiogram Management Urgent surgery, blood pressure control 15