* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Recitation 6 The path of electron flow in photosynthesis from initial
Metalloprotein wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Citric acid cycle wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Photosynthesis wikipedia , lookup
Microbial metabolism wikipedia , lookup
Electron transport chain wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
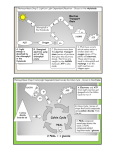
Recitation 6 The path of electron flow in photosynthesis from initial electron donor to final acceptor is sometimes referred to as the Z scheme and is reproduced below from figure 19.22 from your text. As we have seen in class, there are several similarities between oxidative phosphorylation in mitochondria and photosynthesis. For example, both involved a membrane-bound electron transport system, and the mechanisms of ATP production via proton gradients are similar. Electron transport in mitochondria, however, is from a good electron donor, NADH (or FADH2) to a good acceptor, O2. We have already seen how to combine the standard reduction potentials of NAD+ (-0.32 V, which is the same as the reduction potential of NADP +) and O2 (+0.82 V) to get an overall )E0' = +1.14 V, which corresponds to an overall )G0' = -220.1 kJ/mole (using )G = -nF)E) for the overall reaction NADH + ½ O2 + H+ 6 NAD+ + H2O We have also seen that this exergonic reaction can be coupled to the endergonic formation of 3 ATP (2 when FADH2) is the donor, such that the overall reaction is exergonic, or favorable. In the case of photosynthesis, electron flow is from a poor donor to a poor acceptor. In order for downhill, or favorable, flow of electrons to occur, light must be aborbed by the thylakoid-bound pigments which comprise the photosystems depicted above. This, in effect, decreases the reduction potential, thereby increasing the oxidation potential (or the tendency to donate electrons) such that electron flow becomes downhill in the Z scheme, or favorable. It turns out that a total of 4 photons must be absorbed per molecule of donor and acceptor (i.e., per pair of electons): initial donor + 4 photons 6 final acceptor 1. Name the initial electron donor and final acceptor in photosynthesis and determine )G0' for the transfer of electrons fron initial donor to final acceptor in the absence of light absorbtion. The fact that O2 is evolved as a byproduct can be taken as a hint. 2. Now couple in the energy from light absorption of 4 photons of red light of wavelength 700 (by photosystmes I and II) nm to show that by coupling this exergonic process to the endergonic flow of electrons in problem 1, the overall process is favorable. Do this by calculating the )G for light absorption, assuming the energy is entirely convertible to free energy ()G). You need to use the following information: Energy per photon = , = h< = hc/8, because c = <8, where h is Planck’s constant (6.626 x 10-34 J.sec, c = speed of light = 3 x 1010 cm/sec, < is the frequence and 8 is the wavelength of light. Also, 1 nm = 10-7 cm, and Avogadro’s number is N = 6.02 x 1023 photons/mole photons. This is a units problem. 3. As we discussed in class, an alternate path for electron flow results when electrons are diverted from Fd back to the cytochrome bf complex, resulting in a cyclic flow of electrons involving only photosystem I. What does this cyclic electron flow accomplish? Is O2 evolved? 4. The outline of the Calvin cycle from your notes is reproduced below CO2 Ru5P I III Regeneration of catalyst 3PG G3P II The Calvin cycle, like the citric acid cycle, is catalytic in nature. Why is a catalyst necessary? What is the catalyst? As shown above, G3P is converted to Ru5P during phase III, but not all the G3P produced is converted to Ru5P. What else is G3P used for? By what familiar process does this occur? What is the ATP and NADPH requirement for this alternate process? Note that the energy requirement for phase I is 1 ATP per 3PG produced and that for phase II is 1 ATP per 3PG and 1 NADPH for each G3P produced.