* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Relationships of Leptin and Sex Hormones with the Body Mass
Gynecomastia wikipedia , lookup
Sex reassignment therapy wikipedia , lookup
Hypothalamus wikipedia , lookup
Sexually dimorphic nucleus wikipedia , lookup
Testosterone wikipedia , lookup
Hormone replacement therapy (male-to-female) wikipedia , lookup
Hyperandrogenism wikipedia , lookup
Hormone replacement therapy (female-to-male) wikipedia , lookup
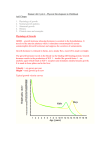
Original Article Relationships of Leptin and Sex Hormones with the Body Mass Index and Waist/Hip Ratio in Aging Taiwanese Males Hung-En Chen, Te-Fu Tsai, Thomas I.S. Hwang Division of Urology, Department of Surgery, Shin Kong WHS Memorial Hospital, Taipei, Taiwan, R.O.C. OBJECTIVE: To evaluate the relationships of leptin and sex hormones with the anthropometric parameters of general obesity (body mass index, BMI) and fat distribution (waist/ hip ratio, WHR). MATERIALS AND METHODS: There were 346 Taiwanese males aged 40~89 (median, 52) 2 years included in this study. The body mass index [BMI, weight (kg)/height (m )] was used as a measure of general obesity. The waist/hip ratio [WHR, waist circumference (cm)/hip circumference (cm)] was used to estimate fat distribution. Leptin and sex steroid levels, including total and free testosterone, dehydroepiandrosterone sulfate (DHEAS), sex hormone-binding globulin (SHBG), and estradiol were assessed using a radioimmunoassay. The relationships of leptin and sex hormones to age-specific ranges (in 10-year increments) were evaluated by the Mann-Whitney test, and these to BMI, WHR, and age were evaluated by use of non-parametric statistics (Pearson coefficients). RESULTS: Free testosterone and DHEAS levels declined with the aging process, showing significant declines in certain age-specific ranges (p < 0.001 to p < 0.05, respectively), and were also inversely correlated with age (r = -0.51, p < 0.001; r = -0.56, p < 0.001, respectively), while an increase of SHBG was related to age (r = 0.38, p < 0.001) and significantly increased in age-specific ranges. Although the BMI and WHR were generally steady among the age-specific ranges (BMI values of between 24.1 ± 3.0 and 25.2 ± 4.4kg/m2, and WHR values of between 0.90 ± 0.04 and 0.93 ± 0.05), WHR demonstrated a significant increase with age (r = 0.28, p < 0.001). Moreover, leptin demonstrated non-significant changes in age-specific ranges, but leptin was shown to be related to age (r = 0.10, p < 0.05) and strongly related to the BMI (r = 0.56, p < 0.001) and WHR (r = 0.28, p < 0.001). CONCLUSIONS: In Taiwanese males, age is accompanied by reductions in sex hormone levels, and increases in SHBG and leptin; moreover, there was no significant change in the fat body mass, but there was a change in the body composition. Only leptin and total testosterone demonstrated significant relationships with age-related changes in anthropometric parameters (BMI and WHR). (JTUA 17:1-7, 2006) Key words: leptin, sex hormone, body mass index, waist/hip ratio, age. The distribution and growth of adipose tissue in men are regulated by both genetic and environmental INTRODUCTION Received: Sep. 21, 2005 Revised: Oct. 30, 2005 Accepted: Feb. 7, 2006 Address reprint requests and correspondence to: Thomas I.S. Hwang, MD Division of Urology, Department of Surgery, Shin Kong WHS Memorial Hospital. No. 95 Wen Chang Rd., Shih Lin District, Taipei, 111, Taiwan, R.O.C. 台灣泌尿醫誌第十七卷第一期(95 年 03 月) 1 Leptin, Sex Hormones and the BMI and WHR Table 1. Leptin and sex-related hormones in normal Taiwanese males (N = 346). Age Leptin Testosterone Free testosterone E2 DHEAS (year) (ng/ml) (ng/ml) (pg/ml) (pg/ml) (ug/dl) 27.2±5.6 179.5±64.0 3.15±1.52 4.92±1.33 40~49(n=171) 12.2±2.97 27.9±6.2 131.3±52.9*** 3.38±1.83 4.66±1.32 50~59(n=103) 10.4±2.53*** 3.10±1.51 4.75±1.52 27.1±6.0 85.6±43.8*** 60~69(n=49) 8.6±2.44*** * 4.16±1.85 4.48±1.93 29.1±6.4 38.3±17.1* ≧ 70(n=23) 7.3±2.38 E2, estradiol; DHEAS, dehydroepiandrosterone sulfate; SHBG, sex hormone-binding globulin. p < 0.05; ** p < 0.01; *** p < 0.001. SHBG (nM) 30.4±13.7 36.5±16.0** 50.9±26.3*** 49.5±20.7 factors. Leptin and sex hormones are hypothesized to significantly affect the male body composition and the quantity of adipose tissue, as well as its distribution in the body [1,2]. However, there are few reports related to aging Taiwanese males [3]. Aging is associated with reductions in plasma sex steroid hormones levels, as well as lower plasma testosterone [4]. Furthermore, a decrease in dehydroepiandrosterone sulfate (DHEAS) levels has also been reported with advancing age [5,6]. Significant changes in body fatness and adipose tissue distribution occur with age, and older men are generally characterized by increased body fatness and preferential accumulations of adipose tissue. Clinically, a steady reduction in adipose tissue found in hypogonadal men in the course of testosterone supplementation has been observed [7], but evidence reveals that the body composition may result in unfavorable alterations of androgen-estrogen activity, especially in aging males [8]. However, to what extent age-related alterations in steroid hormone profiles result from concomitant changes in body composition and increases in adipose tissue is not well documented. Furthermore, the role of leptin, estradiol (E2), and sex hormone-binding globulin (SHBG) on body fatness and its distribution have not been well investigated yet [9], especially in aging Taiwanese males. This study was carried out to evaluate the relationships of leptin and sex hormones with the anthropometric parameters of general adiposity and fat dis- tribution in Taiwanese males; moreover, we compared our data with other reports to evaluate the racial element. were excluded). Data on the quantities of nicotine and alcohol consumed, physical activity, nutritional factors, level of education, profession, and financial status were also taken into consideration. Leptin and sex hormones including total and free testosterone, E2, DHEAS, and sex hormone-binding globulin (SHBG) were assessed using a radioimmunoassay (RIA). Blood samples (5 ml) were drawn after overnight fasting at 08:00-10:00 before any other examination. The reagents used were products of the respective diagnostic kits, such as leptin (LINCO Research, USA), total testosterone (Roche Elecsys, Germany), free testosterone (DSL, USA), DHEAS (Immunotech, France), and SHBG(Immunotech, France). The intra-assay coefficient of variation ranged 4.8% to 6.1%, while the interassay coefficient of variation ranged 5.1% to 10.2%. The BMI [weight (kg)/height (m)2] was used as a measure of general obesity, while the WHR [waist circumference (cm)/hip circumference (cm)] was used to estimate the fat distribution. Among all of the men examined, relationships among hormone levels, BMI, WHR, and age were evaluated by use of non-parametric statistics (Pearson coefficients). Age-specific differences in hormonal variables were evaluated using the Mann-Whitney test. Intergroup differences in anthropometric parameters were evaluated using Student’s t-test. For all tests, p < 0.05 was considered significant. MATERIALS AND METHODS Table 2. The body mass index (BMI) and waist/hip ratio (WHR) of normal Taiwanese males (N = 346). Age BMI WHR (year) (kg/m2) (cm/cm) 24.1±3.0 0.90±0.04 40~49 (n = 171) 25.2±4.4 0.92±0.05* 50~59 (n = 103) 24.9±2.7 0.93±0.05 60~69 (n = 49) 24.3±3.1 0.92±0.04 ≧ 70 (n = 23) p < 0.05. The study cohort was comprised of 346 healthy men aged 40~89 years who went for a physical check-up. The men included in the study suffered from no essential disease (in particular, those connected with gonadal dysfunction) and had no past history of such disease, nor did they present any somatic pathologies on physical examination (in particular, those with hypogonadism 2 JTUA Vol.17 No.1, March 2006 HE Chen, TF Tsai, TIS Hwang Table 3. Relationships of leptin and sex hormones with age, the BMI, and WHR Age BMI WHR r p value r p value r p value Leptin 0.10 < 0.05 0.56 < 0.001 0.27 < 0.001 Sex hormones Total testosterone -0.11 < 0.05 - 0.15 < 0.01 - 0.16 < 0.01 Free testosterone -0.51 < 0.001 - 0.05 > 0.05 - 0.18 < 0.01 DHEAS -0.557 < 0.001 0.08 > 0.05 - 0.17 < 0.01 E2 0.06 > 0.05 0.18 < 0.01 - 0.02 > 0.05 SHBG 0.38 < 0.001 - 0.20 < 0.001 - 0.05 > 0.05 BMI 0.03 > 0.05 WHR 0.28 < 0.001 BMI, body mass index; WHR, waist/hip ratio; E2, estradiol; DHEAS, dehydroepiandrosterone sulfate; SHBG, sex hormone-binding globulin. Fig.1a Free testosterone vs. Age 25 Free testosterone (pg/mL) Coefficient of regression determination: R2 = 0.260 (p < 0.001) Pearson correlation coefficient: r = - 0.51 20 15 10 5 0 40 50 60 70 Fig.1b In this study of 346 Taiwanese middle-aged and elderly males, we found that the aging process had relatively strong inverse relationships with free test- osterone and DHEAS and a positive correlation with SHBG, whereas modest relationships were found with total tes台灣泌尿醫誌第十七卷第一期(95 年 03 月) 90 Leptin vs. vs. Age waist-hip ratio (WHR) DHEAS 10 Coefficient of regression determination: R2 = 0.073 (p < 0.001) 9 correlation coefficient: r = 0.27 Coefficient of regression determination:Pearson R2 = 0.3211 (p < 0.001) 8 Pearson correlation coefficient: r = - 0.557 7 6 5 4 3 2 1 0 400 350 300 250 200 150 100 50 0 40 0.80 50 0.85 60 Age (years) DISCUSSION 80 Age (years) Leptin (ng/mL) Serum levels of leptin and sex-related hormones of essentially normal Taiwanese males in age-specific ranges are listed in Table 1, which reveals the influence of age. Generally, declines in free testosterone and DHEAS with age and a rise in SHBG with age were observed and significantly differed among each 10-year range. No significant changes were found in total testosterone, leptin, or E2 levels among the age-specific ranges. The BMI and WHR of normal Taiwanese males are shown in Table 2. Generally no significant differences between age groups were found for BMI and WHR. The relationships of leptin and sex hormones to age, BMI, or WHR are shown in Table 3. Trends of reduced total testosterone (r = -0.11, p < 0.05), free testosterone (r = -0.51, p < 0.001) and DHEAS (r = -0.56, p < 0.001) levels were found with age (Fig. 1a, b), while trends of increased SHBG (r = 0.38, p < 0.001) and leptin (r = 0.10, p< 0.05) levels were related to age. The WHR was related to age (r = 0.28, p < 0.001), while the BMI was not. Moreover, only leptin was shown simultaneously to be strongly related to the BMI (r = 0.56, p < 0.001) and WHR (r = 0.27, p < 0.001) (Fig. 2a, b), while total testosterone was inversely and modestly related to the BMI and WHR (r = -0.15, p < 0.01, r = -0.16, p < 0.01, respectively) (Table 3). tosterone and leptin, but no relation was found with E2. Our results support most studies which have reported an aging influence on sex hormones in healthy males [1,4,10]. DHEAS (ug/dL) RESULTS 0.90 70 0.95 80 1.00 90 WHR (cm/cm) Fig 1. Relationships of free testosterone and dehydroepiandrosterone sulfate (DHEAS) with age. The curves reveal that free testosterone and DHEAS decline with age (1a, r= -0.51, p < 0.001; 1b, r = -0.557, p < 0.001). Leptin is a protein hormone produced by adipose 3 1.05 Leptin, Sex Hormones and the BMI and WHR 4 Fig.2a 12 Leptin vs. body-mass index (BMI) Coefficient of regression determination: R2 = 0.304 (p < 0.001) Pearson correlation coefficient: r = 0.56 10 Leptin (ng/mL) cells, and it modulates food intake and the metabolic rate [1]. Leptin predominately influences human energy balance through neuronal pathways by regulating food intake and may interact with other hormones, such as testosterone, ghrelin, and adeponectin, in regulating energy expenditures, although the exact mechanism is still unclear [11,12].Leptin levels significantly increased with age in Taiwanese middle-aged and elderly males in this study as has been reported in Caucasian males [11,13], while Perry et al. reported that leptin levels tended to be inversely correlated with age (r = -0.228, p = 0.09) in African-American men [10]. In most studies, leptin is highly correlated with body fat and the BMI [10,14,15]. In this study, leptin levels were found to be strongly correlated with the BMI and WHR among Taiwanese males, although there are fewer obviously and extremely obese individuals among Taiwanese compared to Western people who maintain much higher BMI and WHR values [10,15]. What is the significance of the strong correlation of leptin with the BMI and WHR? After cloning of the ob gene, the severely obese leptin-deficient ob/ob mice were injected with leptin, which led to weight loss [16]. These findings support the initial concept that leptin’s function is to control weight gain by reducing food intake and increasing energy expenditure, and that human obesity might also be a leptin-deficient state. However, obese homozygous individuals with a leptin deficiency state have only rarely been found, and several demographic studies have failed to demonstrate a mutation in the human gene coding for leptin. In contrast, elevated serum levels of leptin in obese individuals have been found [17]. It is clear now that the vast majority of human obesity is associated with increased levels of leptin. A similar result was found in our study. Although the exact mechanism is not clear, a hypothesis of leptin resistance or reduced sensitivity to leptin associated with human obesity is suggested by these findings. The BMI is used as a reliable measure of general obesity and has been strongly related to a variety of measures of fatness [18], especially among middle-aged adults. Obesity is a heterogeneous condition, and not every obese patient is at an increased risk of cardiovascular disease; only an unfavorable fat distribution (such as visceral obesity) is a critical complication of obesity [6]. The WHR was used to estimate visceral obesity, which is considered an increased risk for cardiovascular complications [6]. Moreover, this type of obesity is associated with several hormonal and metabolic aberrations, as well as increased risks of developing cardiocerebrovascular disease and non– insulin -dependent diabetes mellitus [18]. In men, testosterone is the main testicular androgen 8 6 4 2 0 15 20 25 30 35 BMI (kg/m2) Fig.2b Fig 2. Relationships of leptin with the body mass index (BMI) and waist/hip ratio (WHR). The curves reveal that leptin is positively correlated with both the BMI (2a, r = 0.56, p < 0.001) and WHR ( 2b, r = 0.27, p < 0.001). [19], E2 is the main circulating estrogen, and DHEAS is the main adrenal androgen [6,19], whereas SHBG is a binding protein which regulates the tissue availability of free androgens and estrogens [20]. In most populations of elderly men, BMI has been associated with decreases in SHBG [18,20], DHEAS [4,6,14], total testosterone and, free testosterone [4,20], and increases in E2 [10,13]. However, only modest inverse correlations of total testosterone and the BMI (r = -0.15, p < 0.01), and SHBG and the BMI (r = -0.20, p < 0.001), and a positive correlation of E2 and the BMI (r = 0.18, p < 0.01) were found in Taiwanese males in this study, while no correlations were demonstrated for free testosterone or DHEAS with the BMI. Visceral adiposity has been generally linked to adrenal and gonadal hypoandrogenicity in men[7]. Some studies have confirmed an inverse association of the WHR with total and free testosterone levels as well as with DHEAS [15], as did this study (Table 3). However, another study found no correlation with testosterone and JTUA Vol.17 No.1, March 2006 HE Chen, TF Tsai, TIS Hwang DHEAS [4], in healthy or in obese men, suggesting the extent of decline in sex hormones mainly depends on the degree of obesity. 8. CONCLUSIONS Significant correlations of leptin with the BMI as well as with the WHR were demonstrated in Taiwanese males, while only a modest inverse correlation of total testosterone to BMI and inverse correlations of total and free testosterone to WHR were shown. This study suggests that androgen decline caused by normal aging is not obviously related to general obesity or body composition in older Taiwanese males. Therefore, androgen supplementation in hypogonadal older men is not expected to influence body composition to the same extent. ACKNOWLEDGEMENTS This study was supported by a research grant (8302-90-1601-01) from Shin Kong WHS Memorial Hospital; technical assistance by Ms. Karen Zu-Hwa Cheng is gratefully acknowledged. REFERENCES 1. 2. 3. 4. 5. 6. 7. Björntorp P. Adipose tissue distribution and function. Int J Obesity 1991;15:67-81. Tchernof A, Despres JP, Belanger A, et al. Reduced testosterone and adrenal C19 steroid level in obese men. Metabolism 1995;44:513-9. Wu CY, Yu TJ, Chen MJ. Age related testosterone level changes and male andropause syndrome. Chang Gung Med J 2000;23:348-53. Denti L, Pasolini G, Sanfelici L, et al. Aging-related decline of gondal function in healthy men: correlation with body composition and lipoproteins. J Am Geriatr Soc 2000;48:51-8. Labrie F, Belanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentration of adrenal C19, sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endo- crinol Metab 1997;82:2396-402. Tchernof A, Labrie F, Belanger A, Despres JP. Obesity and metabolic complications: contribution of DHEA and other steroid hormones. J Endocrinol 1996;150(Suppl):S155-64. Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypo- 台灣泌尿醫誌第十七卷第一期(95 年 03 月) 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. gonadism. J Clin Endocrinol Metab 1996; 81: 4358-65. Couillard C, Gagnon J, Bergeron J, et al. Contribution of body fatness and adipose tissue distribution to the age variation in plasma steroid hormone concentrations in men: the HERITAGE family study. J Clin Endocrinol metab 2000;85: 1026-31. Tritos NA, Mantzoros CS. Leptin: its role in obesity and beyond. Diabetologia 1997;40:1371-9. Perry HM III, Miller DK, Patrick P, Morley JE. Testosterone and leptin in older African-American men: relationship to age, strength, function and season. Metabolism 2000;49:1085-91. Luukkaa V, Pesonen U, Huhtaniemi I, et al. Inverse correlation between serum testosterone and leptin in men. J Clin Endocrinol Metab 1998;83:3243-6. Yildiz BO, Suchard MA, Wong ML, McCann SM, Licinio J. Alternations in the dynamics of circulating ghrelin, adeponectin, and leptin in human obesity. PNAS 2004;101:10434-9. Behre HM, Simoni M, Nieschlag E. Strong association between serum levels of leptin and test- osterone in men. Clin Endocrinol (Oxford) 1993; 43:237-40. Field AE, Colditz GA, Willett WC, Longcope C, McKinlay JB. The relation of smoking, age, relative weight, and dietary intake to serum adrenal steroids, sex hormones, and sex hormone-binding globulin in middle-aged men. J Clin Endocrinol Metab 1994; 79:1310-16. Jankowska EWA, Medras M, Rogucka EA. Body mass index, waist/hip ratio and antrogen-estrogen activity in younger versus older Polish men. Aging Male 2000;3:177-84. Halaas JL, Gajiwala KS, Maffei M, et al. Weightreducing effects on the plasma protein encoded by the obese gene. Science 1995; 269: 543-46. Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996;334:292-95 Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med 1999;341:1097-105. Pasquali R, Casimirri F, Cantobelli S, et al. Effect of obesity and body fat distribution on sex hormones and insulin in men. Metabolism 1991; 40:101-4. Parker LN. Control of adrenal androgen secretion. Endocrinol Metabol Clin N Am 1991;20:410-21. 5 Original Article Received: Sep. 21, 2005 Revised: Oct. 30, 2005 Accepted: Feb. 7, 2006 Address reprint requests and correspondence to: Thomas I.S. Hwang, MD Division of Urology, Department of Surgery, Shin Kong WHS Memorial Hospital. No. 95 Wen Chang Rd., Shih Lin District, Taipei, 111, Taiwan, R.O.C. 台灣泌尿醫誌第十七卷第一期(95 年 03 月) 1