* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cyber-physical modeling of implantable cardiac medical devices
Coronary artery disease wikipedia , lookup
Heart failure wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Myocardial infarction wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Cardiac surgery wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
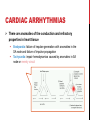
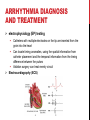
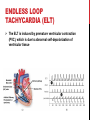
CYBER-PHYSICAL MODELING OF IMPLANTABLE CARDIAC MEDICAL DEVICES SOL YOON ICE, DGIST FEB. 6 TH . 2012 OUTLINE Introduction Overview of model-based design Background knowledge Integrated Heart Model Heart Model Validation Pacemaker Model Closed-loop case study Conclusion INTRODUCTION THE FDA AND MEDICAL DEVICE SOFTWARE FDA: need for rigorous real-time methodologies to validate and verify medical device software The use of artificial implantable heart rhythm devices has grown rapidly over the recent decades However, there is no formal methodology or platform to validate and verify the correct operation of medical device software Software is reviewed by the FDA only in the incident of a device recall. Implantable medical devices are a primary example of medical cyber-physical systems Safety and efficacy of the device and device software must be evaluated within a closed-loop context of the patient CURRENT TESTING, VALIDATION, AND VERIFICATION The primary approach is unit testing using a playback of prerecorded electrogram and electrocardiogram signals April 2010, the FDA began the “Infusion Pump Improvement Initiative” An effective verification methodology is needed for the risk analysis and certification of medical device software Pacemaker mediated tachycardia (PMT) A condition where the pacemaker inappropriately drives the heart rate toward the upper rate limit Can be used for closed-loop system analysis METHODOLOGY FOR CLOSEDLOOP MEDICAL DEVICE SAFETY Developed an integrated functional and formal virtual heart model (VHM) Clinically relevant Timed automata based Developed a pacemaker device model for interactive and clinically relevant test generation A set of general and patient condition-specific pacemaker software requirement to ensure the safety Provide a means to test and verify the closed-loop system The atrial-ventricle synchrony must be enforced OVERVIEW OF MODEL-BASED DESIGN PREVIOUS HEART MODELING EFFORTS The model of the heart should capture the electrophysiological (EP) properties of the heart and generate functional signals Conduction Timing signal The heart models have been developed to study the heart functions from the electrical and mechanical aspects. Signal propagation, distortion, and attenuation Cardiac output and valve mechanisms REQUIREMENTS FOR MODELBASED CLOSED-LOOP V&V 1. Model fidelity: must cover the functioning heart Normal sinus rhythm, sinus bradycardia, pacemaker mediated tachycardia, etc. 2. Simplicity The heart model currently have hundreds of differential equations or millions of finite elements Simulation of the models are time consuming and do not interact with medical devices The VHM presents an abstraction of the timing and electrical conduction 3. Physical testbed Enable to operate the heart on VHDL-based FPGA platform for blackbox closed-loop testing OVERVIEW OF THE VHM APPROACH Platform provide two interface A formal signal for medical device software A functional electrogram for real device implementation BACKGROUND KNOWLEDGE BACKGROUND KNOWLEDGE The heart generates electrical impulses which organize the sequence of muscle contractions during each heart beat The heart’s electrical timing is fundamental to proper cardiac function The implantable cardiac pacemaker is a rhythm management device Such devices have improved the condition of patients with cardiac arrhythmias CELLULAR-LEVEL ACTION POTENTIAL The heart tissue can be activated by an external voltage applied to the cell ELECTRICAL CONDUCTION SYSTEM The tissue at the sinoatrial node (SA) periodically and spontaneously self-depolarizes The activation signal travels through both atria, causing contraction and pushes blood into the ventricles. Then the activation is delayed at the atrioventricular (AV) node which allows the ventricles to fill fully CARDIAC ARRHYTHMIAS There are anomalies of the conduction and refractory properties in heart tissue Bradycardia: failure of impulse generation with anomalies in the SA node and failure of impulse propagation Tachycardia: impair hemodynamics caused by anomalies in SA node or reentry circuit ARRHYTHMIA DIAGNOSIS AND TREATMENT electrophysiology (EP) testing Catheters with multiple electrodes on the tip are inserted from the groin into the heart Can locate timing anomalies, using the spatial information from catheter placement and the temporal information from the timing difference between the pulses Ablation surgery can treat reentry circuit Electrocardiography (ECG) RHYTHM MANAGEMENT DEVICES Implantable pacemakers have been developed to deliver timely electrical pulses to the heart to treat bradycardia The pacemaker has two leads inserted into the heart One in the right atrium One in the right ventricle By doing timing analysis of the electrogram (EGM) signals sensed from the two leads Artificial pacemaker generates electrical pulses when necessary that can maintain ventricular rate Enforce atrial-ventricular synchronization HEART MODEL A BRIEF OVERVIEW OF EXTENDED TIMED AUTOMATA VHM uses a timed-automata semantics, which is similar to the semantic extension used in UPPAAL The electrical conduction system consists of conduction pathways with different conduction delays and refractory period The refractory and conduction properties are all timing based, it is natural to model the electrical conduction system as a network of timed automata MODELING THE ELECTRICAL CONDUCTION SYSTEM (a) Node automaton that models the refractory properties of heart tissue 𝑡 𝑓 𝑡 = 1 − 𝑇𝑟𝑟𝑝 , 𝑡 ≥ 1, 𝑡 ≥ 𝑇 𝑇𝑚𝑖𝑛 + (1 − (1 − 𝑥)3 ) ∙ 𝑇𝑚𝑎𝑥 − 𝑇𝑚𝑖𝑛 , 𝑖 = 𝐴𝑉 𝑔 𝑥 = 𝑇𝑚𝑖𝑛 + (1 − 𝑥)3 ∙ 𝑇𝑚𝑎𝑥 − 𝑇𝑚𝑖𝑛 , 𝑖 ≠ 𝐴𝑉 𝑇𝑚𝑖𝑛 and 𝑇𝑚𝑎𝑥 are the minimum and maximum values for 𝑇𝑒𝑟𝑝 of the tissue MODELING THE ELECTRICAL CONDUCTION SYSTEM (b) Path automaton that models the propagation properties of heart tissue 𝑝𝑎𝑡ℎ_𝑙𝑒𝑛/𝑣 ∙ 1 + 3𝑐 , 𝑖 = 𝐴𝑉 𝑝𝑎𝑡ℎ_𝑙𝑒𝑛/𝑣 ∙ 1 + 3𝑐 2 , 𝑖 ≠ 𝐴𝑉 ℎ 𝑐 = 𝑝𝑎𝑡ℎ_𝑙𝑒𝑛 denote the length of the path and vis the conduction velocity HEART MODEL VALIDATION ELECTROPHYSIOLOGY STUDY 1. Catheter Placement The typical catheter positions used are high right atrium (HRA) His bundle electrogram (HBE), which is placed across the valve between atrium and ventricle Right ventricle apex (RVA), which is placed at the right ventricle apex to monitor electrical activity of the ventricle 2. Extrastimuli Technique HRA catheter deliver external pacing signals faster than the intrinsic heart rate The interval between two consecutive pacing signals is referred to as basic cycle length (BCL) The interval between the extrastimulus and the last pacing signal of the pacing sequence is referred to as coupling interval By decreasing the coupling interval gradually, the extrastimulus will reach the RRP of the tissue, causing changes in conduction delays CLINICAL CASE STUDY Key interval values when the coupling interval shortens for a real patient 𝐴2 , 𝐻2 , 𝑉2 are the pulse caused by the extrastimulus The interval 𝐴1 − 𝐴2 is equal to the coupling interval 𝐻1 − 𝐻2 , 𝑉1 − 𝑉2 indicate conduction delay between the His bundle and the ventricle Caused by extrastimulus Coupling interval VHM SIMULATION VHM is able to generate similar result with extrastimuli technique PACEMAKER MODEL PACEMAKER MODEL The artificial pacemaker is designed for patients with bradycardia Two leads, one in the right atrium and one in the right ventricle, are inserted into the heart Two leads monitor the local activation of the atria and the ventricles, and generate corresponding sensed event (AS, VS) to its software The software determines the heart condition by measuring time difference between events and delivers pacing events (AP, VP) to analog circuit Analog circuit delivers pacing signals to the heart to maintain heart rate and A-V synchrony DDD PACEMAKER TIMING DIAGRAM Five basic timing cycles which diagnose heart condition Ventricle pace (LRI), ventricle sense (AVI) Atrial pace (ARP), atrial sense (VRP) Coordinator between the atrium and ventricle leads (URI) Each task was assigned a period of 10 ms CLOSED-LOOP CASE STUDY ENDLESS LOOP TACHYCARDIA (ELT) The ELT is induced by premature ventricular contraction (PVC), which is due to abnormal self-depolarization of ventricular tissue CONCLUSION PHYSICAL IMPLEMENTATION Can validate the closed-loop electrical interaction between the heart (FPGA) and pacemaker (FireFly node) CONCLUSION AND FUTURE WORK A primary challenge in life-critical real-time systems is with the design of bug-free medical device software Using timed automata designed an integrated functional and formal model of the heart and pacemaker device A real-time VHM has been developed to model the electrophysiological operation of the human heart