* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Combinatorial Gli gene function in floor plate and
Survey
Document related concepts
Transcript
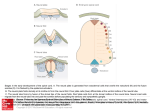
2203 Development 125, 2203-2212 (1998) Printed in Great Britain © The Company of Biologists Limited 1998 DEV6336 Combinatorial Gli gene function in floor plate and neuronal inductions by Sonic hedgehog A. Ruiz i Altaba The Skirball Institute, Developmental Genetics Program and Department of Cell Biology, NYU Medical Center, 540 First Avenue, New York, NY 10016, USA *Author for correspondence: (e-mail: [email protected]) Accepted 2 April; published on WWW 19 May 1998 SUMMARY Within the developing vertebrate nervous system, it is not known how progenitor cells interpret the positional information provided by inducing signals or how the domains in which distinct groups of neural cells differentiate are defined. Gli proteins may be involved in these processes. In the frog neural plate, we have previously shown that the zinc finger transcription factor Gli1 is expressed in midline cells and mediates the effects of Shh inducing floor plate differentiation. In contrast, Gli2 and Gli3 are expressed throughout the neural plate except for the midline. Here, it is shown that Gli3 and Shh repress each other whereas Gli2, like Gli1, is a target of Shh signaling. However, only Gli1 can induce the differentiation of floor plate cells. In addition, Gli2 and Gli3 repress the ectopic induction of floor plate cells by Gli1 in co-injection assays and inhibit endogenous floor plate differentiation. The definition of the floor plate domain, therefore, appears to be defined by the antagonizing activities of Gli2 and Gli3 on Gli1 function. Because both Gli1 and Gli2 are induced by Shh, these results establish a regulatory feedback loop triggered by Shh that restricts floor plate cells to the midline. We have also previously shown that the Gli genes induce neuronal differentiation and here it is shown that there is specificity to the types of neurons the Gli proteins induce. Only Gli1 induces Nkx2.1/TTF-1+ ventral forebrain neurons. Moreover, Gli2 and Gli3 inhibit their differentiation. In contrast, the differentiation of spinal motor neurons can be induced by the two ventrally expressed Gli genes, Gli1 and Gli2, suggesting that Gli2 directly mediates induction of motor neurons by Shh. In addition, Gli3 inhibits motor neuron differentiation by Gli2. Thus, combinatorial Gli function may pattern the neural tube, integrating positional information and cell type differentiation. INTRODUCTION To analyze the molecular mechanisms involved in the interpretation of positional information, we have focused on the function of the Gli gene family (Kinzler et al., 1987; Ruppert et al., 1988, 1990; Walterhouse et al., 1993; Hui et al., 1994; Marigo et al., 1996; Lee et al., 1997; Marine et al., 1997; Platt et al., 1997; Hughes et al., 1997) which includes cubitus interruptus (ci; Orenic et al., 1990; Eaton and Kornberg, 1990) in Drosophila. In frog embryos, Gli1 is expressed in midline cells at the time that they are induced to become floor plate and in cells lateral to the midline that appear to differentiate into primary motor neurons (Lee et al., 1997). Gli2 is expressed throughout the neural plate with the exception of the midline and Gli3, also absent from the midline, shows a graded expression with highest levels laterally. We have shown that Gli1, but not Gli3, is a target of Shh and that it can mediate floor plate induction by Shh (Lee et al., 1997). Consistent with these results, Gli1 also induces ectopic ventral development in mice (Hynes et al., 1997) and mimics Shh signaling in the chick limb (Marigo et al., 1996). Gli proteins show a conserved five zinc finger domain with highest identity in the last three fingers, which bind DNA (Pavletich and Pabo, 1993). Indeed, A critical question in understanding the formation of the vertebrate nervous system is how the mechanisms of positional information interface with the mechanisms of neurogenesis and gliogenesis to yield a patterned program of neural cell differentiation. Within the early frog neural plate, this problem can be defined as how the floor plate attains its identity and how its domain is restricted to the midline, whereas distinct types of neurons differentiate at defined positions within the neural plate. Mediolateral (M-L), future dorsoventral (D-V), pattern develops partly as the result of positional information provided by the graded and antagonizing actions of Sonic hedgehog (Shh), from the notochord and floor plate (Riddle et al., 1993; Echelard et al., 1993; Krauss et al., 1993; Roelink et al., 1994, 1995; Ruiz i Altaba et al., 1995; Ekker et al., 1995; Martí et al., 1995; Ericson et al., 1995, 1996; Hynes et al., 1995; Chiang et al., 1996), and Bone Morphogenetic Proteins (BMPs), derived initially from the inducing epidermal ectoderm (Moury and Jacobson, 1989; Liem et al., 1995; Dickinson et al., 1995). Key words: Gli, Sonic hedgehog, Floor plate, Induction, Pattern formation, Gene function, Neuron, Zinc finger 2204 A. Ruiz i Altaba Gli proteins recognize the same target sites in vitro (Kinzler and Vogelstein, 1990; Vortkamp et al., 1995; Sasaki et al., 1997; Marine et al., 1997). Finally, all three Gli proteins have a strong neurogenic effect and Gli function is involved in primary neurogenesis (Brewster et al., 1998). Here, the effects of Gli proteins on neural patterning are examined and it is shown that the Gli genes respond to Shh and induce distinct programs of neural cell differentiation. The results suggest that the definition of the domains of floor plate and ventral forebrain neuronal differentiation involve a regulatory feedback loop in which Gli2, which is induced by Shh at a distance from midline cells, acts to restrict the activity of Gli1 to a ventral domain. In addition, Gli2 appears to mediate the induction of motor neurons by Shh. The restriction of motor neuron differentiation by Gli2 to the ventral region may be due to an inhibitory effect of Gli3, the expression of which overlaps with that of Gli2 in dorsal regions. Together, these results suggest that the Gli proteins interact in partially overlapping and partially antagonizing ways to establish spatial pattern and cell identity in the developing vertebrate nervous system. RNA probes were made as follows: a Shh cDNA clone (Ruiz i Altaba et al., 1995) was digested with NotI and transcribed with T3. A Pax3 cDNA clone (Kim et al., 1997) was digested with EcoRI and transcribed with SP6. An xHB9 cDNA (Saha et al., 1997) was digested with NotI and transcribed with T7. RNA probes for frog and mouse Gli1, Gli2 and Gli3 and mouse Shh were as described (Lee et al., 1997). Labeling frog embryos with anti-HNF-3β (Ruiz i Altaba et al., 1995; Lee et al., 1997) or anti-Nkx2.1/TTF-1 polyclonal antibodies (Lazzaro et al., 1991) by whole-mount immunocytochemistry with peroxidase-DAB/H2O2 was as described (Lee et al., 1997). Histological sections (10-15 µm) of Paraplast-embedded embryos were cut in a microtome and mounted in Permount. RESULTS The Gli genes respond to Shh signaling Gli1 is expressed in midline neural plate cells (Fig. 1A) and then in immediately adjacent cells (Fig. 1B), which appear to be early differentiating primary motor neurons (Lee et al., 1997). In contrast, Gli2 and Gli3 are expressed widely in the neural plate but not at the midline (Fig. 1C,E), with Gli3 showing a graded MATERIALS AND METHODS Embryos and microinjection Xenopus laevis embryos were obtained and reared by standard techniques and staged according to Nieuwkoop and Faber (1967). Microinjection of plasmid DNA (100-200 pg/10 nl/embryo) or synthetic RNA was performed by standard techniques (e.g. Ruiz i Altaba, 1993) into one cell at the 1- to 2-cell stage. RNA injections were at 2 ng/10 nl/embryo unless otherwise indicated. Co-injections were performed at 1 ng for each RNA (2 ng total), and single injections serving as controls for the co-injections were performed at 1 ng/embryo also thus maintaining the same amount of tested RNA (e.g. Gli1 or Gli2). Albino embryos were routinely used for injection. For mouse embryos E0.5 was counted as the morning when the plug was found. Plasmid clones The vectors used for injection into frog embryos were pcDNA1-Amp (Invitrogen) or pCS2 and pCS2-myc tag (Turner and Weintraub, 1994; Rupp et al., 1994). All vectors used contained CMV regulatory elements, which provide ubiquitous expression of human (h) or frog (f) cDNAs. Plasmids expressing hGli1, hGli3 and fGli1 were as described previously (Lee et al., 1997). Plasmids driving the expression of fShh were as described in Ruiz i Altaba et al. (1995). The hGli1-hGli3 chimeric construct was made by replacing the Nterminal region (bp 1-1248) of hGli3 with a 702 bp Pwo PCR Nterminal fragment of hGli1, in pcDNA1-Amp. The swap point was immediately upstream of the zinc finger DNA-binding domain in both hGli1(at bp 702, aa 208) and hGli3 (at bp 1248, aa 398). The internal zinc finger deletion of hGli3 was obtained by linearization with BglII followed by controlled digestion with Bal31 and religation. The inframe deletion (504-643) removes the zinc finger DNA-binding domain with the exception of the first 22 aa of finger 1. fGli2 and fGli3 cDNAs derived from our initial Gli screen (Lee et al., 1997) and were as described (Brewster et al., 1998). Synthetic RNAs for injection were made by transcription of pCS2 constructs with SP6 polymerase after digestion with NotI in the presence of cap analog. Reactions were done at 30°C to enhance production of full-length 4-5 kb transcripts. In situ hybridization and immunocytochemistry Whole-mount in situ hybridization was carried out as described by Harland (1991) using maleic acid. Antisense digoxigenin-labeled Fig. 1. Neural Gli gene expression, induction of Gli2 and repression of Gli3 by Shh. (A,B) Expression of Gli1 in midline (md) cells of a late gastrula/early neurula embryo (A; stage ~12) and in immediately adjacent cells in older neurulae (B; stage ~14). (C) Expression of Gli2 in a neural plate stage embryo (stage 13; n=45). Note its absence from the midline. (D) Ectopic expression of Gli2 (arrows) in a stage ~13 embryo injected with Shh plasmids (88% n=34). Ectopic expression of Gli2 was also induced by hGli1 (27% n=11) or VP16fGli1 (38% n=21; not shown). (E) Expression of Gli3 at the early neurula stage (stage ~13, n=23) in the neural folds (nf). Inset shows cross section of a stage 14 embryo showing a graded medial to lateral expression. (F) Expression of Gli3 in an embryo (stage ~13) injected with Shh plasmids (32% n=40) with unilateral repression of Gli3 (arrows). Repression of Gli3 was also observed after injection of hGli1 (20% n=20) or fGli1 (30% n=10; not shown). (A) Lateral/posterior view; (B,C,E,F) dorsal views with anterior end up; (D) lateral view with dorsal side to the left and anterior end up. Dorsal side is up for the section in the inset of E. Dashed line depicts the axis of bilateral symmetry. md, midline cells. Pattern formation by combinatorial Gli gene function 2205 distribution along the M-L axis (Fig. 1E inset). Because Gli1 is a target of Shh in the neural plate (Lee et al., 1997) and Gli3 is repressed by Shh in the chick limb (Marigo et al., 1996), we tested for the effects of Shh on the expression of Gli2 and Gli3. Frog embryos injected with plasmids driving the expression of Shh displayed the ectopic, albeit mosaic, expression of Gli2 in the ectoderm (Fig. 1D). In contrast, Shh repressed Gli3 transcription (Fig. 1F). Similar results were obtained by injecting Gli1 (not shown), consistent with the role of Gli1 as a mediator of Shh signaling (Lee et al., 1997). Gli3 mutant embryos display ectopic Shh expression in the dorsal neural tube The ability of Shh to repress Gli3 in the neural plate together with the ectopic expression of Shh in anterior limb regions of Gli3 mutant mice (Masuya et al., 1995), raised the possibility that this mutual antagonistic interaction between Gli3 and Shh is also operative in the neural tube. In the spinal cord of E12.5 mouse embryos, Shh is expressed in the floor plate (Fig. 2A), Gli1 is expressed in the ventral ventricular zone immediately dorsal to the floor plate (Fig. 2C; Lee et al., 1997; Sasaki et al., 1997) and Gli2 and Gli3 are expressed in the dorsal ventricular zone, with Gli2 also showing low levels of expression in the ventral ventricular zone (Fig. 2D and not shown; Lee et al., 1997; Sasaki et al., 1997). Extra-toes (XtJ) mutant mice have an intragenic deletion of Gli3 and show overt defects in brain development (Hui and Joyner, 1993; Franz, 1994). E12.5 extratoes mutant embryos were found to display moderate levels of ectopic Shh expression in the dorsal ventricular zone of the neural tube, the region that normally expresses Gli2 and Gli3 (Fig. 2C and not shown: Hui et al., 1994; Lee et al., 1997; Sasaki et al., 1997). Little change was observed in XtJ mice for Gli2 expression whereas Gli1 was ectopically expressed at low levels (not shown). Ectopic gene expression in XtJ mice, however, is a variable phenotype and was detected in under 30% of tested homozygote −/− embryos. Such variability has also been reported for marker expression in the limb buds of Xt mice (Masuya et al., 1995). The near normal morphology of the spinal cord in some Gli3 mutant mice suggests that other factors, in addition to Gli3, regulate Shh. Nevertheless, Gli3 appears to be a general repressor of Shh. Table 1. Quantitation of injection results (A) Ectopic induction of HNF-3β+ cells by Gli1 but not Gli2 or Gli3 pDNA Marker Injection Ectopic Normal n HNF-3β " " " " " " fGli1 hGli1 fGli2 fGli3 hGli3 hGli1→hGli3 control 98 72 5 0 6 49 2 2 28 95 100 94 51 98 100 190 40 30 170 72 42 (B) Antagonistic action of Gli2 and Gli3 on ectopic HNF-3β induction by Gli1 Marker Injection Ectopic Normal n pDNA HNF-3β " " " " " " " " hGli1 " + hGli3 " + hGli3zf∆ " + fGli2 45 6 60 17 55 94 40 83 42 150 148 53 fGli1 " + hGli3 " + hGli3zf∆ " + fGli2 60 28 40 24 40 72 60 76 60 28 50 66 (C) Differential effects of Gli proteins on endogenous HNF-3β expression RNA Fig. 2. Expression of Shh, Gli1 and Gli2 in wild-type mouse embryos and ectopic Shh expression in Gli3 mutant embryos. (A) Expression of Shh RNA in the floor plate (fp) in the thoracic spinal cord of wildtype (+/+) E12.5 mice. (B) Ectopic expression of Shh in the dorsal ventricular zone of E12.5 Gli3 mutant (−/−) mice. The ectopic expression of Shh in the ventricular zone mimics the normal expression of Gli2 (D) and Gli3 (Lee et al., 1997; Sasaki et al., 1997). (C) Expression of Gli1 in the spinal cord of E12.5 wild-type mice. Note the focal expression in the ventral ventricular zone (vvz). (D) Expression of Gli2 in a wild-type E12.5 spinal cord. High levels are present in the dorsal ventricular zone (dvz) and lower and more restricted expression is detected in the ventral ventricular zone (vvz). All panels show cross sections with dorsal side up. Marker Injection Ectopic Repressed Normal n HNF-3β " " " " " fGli1 fGli2 fGli3 hGli3 " D2/4 hGli3-zf∆ 100 0 0 0 0 0 0 14 20 50 81 0 0 86 80 50 19 100 18 14 14 12 32 45 (D) Reciprocal rescue of the effects of Glis on HNF-3β expression RNA Marker Injection HNF-3β " " " " " fGli1 " + fGli3 " + hGli3 " + hGli3zf∆ " + fGli2 control Ectopic Repressed Normal n 100 16 19 93 23 0 0 0 14 0 4 0 0 84 67 7 73 100 48 42 47 46 52 61 (A-D) Summary of injection results as indicated. All numbers refer to percentages except the total number of embryos (n). D2/4 in C signifies injection into both dorsal blastomeres at the 4-cell stage. See text for details. 2206 A. Ruiz i Altaba Gli1, but not Gli2 or Gli3, can induce floor plate cell differentiation To test the effects of injected Gli proteins (Table 1A), the expression of HNF-3β has been used as a marker of floor plate cell differentiation in early tadpoles (Fig. 3A; Ruiz i Altaba et al., 1995; Lee et al., 1997). Expression of Gli1 proteins from injected plasmids or synthetic RNA resulted in ectopic expression of HNF-3β within the neural tube (Fig. 3B,C; Table 1B,D). Neither injection of Gli2 nor Gli3 resulted in ectopic HNF-3β expression (Table 1B,D; Lee et al., 1997). These results show that Gli proteins have non-redundant functions as only Gli1 can induce floor plate differentiation. Gli proteins appear to recognize the same target genes in vivo Because both human Gli1 and Gli3 bind to the same DNA sequences in vitro (Kinzler and Vogelstein, 1990; Vortkamp et al., 1995; Sasaki et al., 1997), a chimeric Gli3 protein was constructed having its N-terminal region replaced by that of Gli1, retaining the zinc finger DNA-binding and C-terminal domains of Gli3 (Table 1A). Injection of this hGli1→hGli3 chimera resulted in ectopic expression of HNF-3β, a Gli1 target gene, albeit at lower frequency than hGli1 (Table 1B). The Nterminal part of hGli1 can therefore confer floor-plate-inducing activity to hGli3 and suggests that Gli proteins can recognize the same DNA targets in vivo. Gli2 and Gli3 antagonize ectopic floor plate induction by Gli1 If all Gli proteins bind the same targets, the inability of Gli2 and Gli3 to activate HNF-3β indicates that they could act as repressors in vivo, consistent with the repressor activity of Gli3 in vitro (Marine et al., 1997; Sasaki et al., 1997). To investigate whether Gli2 or Gli3 could repress Gli1 function, plasmids driving the expression of Gli1 and Gli3 or Gli1 and Gli2 were co-injected into developing frog embryos. Gli2 and Gli3 were able to repress the ectopic HNF-3β-inducing activity of frog or human Gli1 (Table 1C), a nuclear-targeted Gli1 and a VP16-Gli1 derivative (not shown; Lee et al., 1997). Injection of a Gli3 mutant construct with an in-frame deletion of the zinc finger DNA-binding domain was ineffective (Table 1C). Together, these results show that Gli2 and Gli3 lack the ability to induce floor plate development and that they antagonize the effects of Gli1, possibly by competition for regulatory sites in target genes. Gli2 and Gli3 repress endogenous floor plate development The effects of ectopic Gli3 and Gli2 expression in the ventral neural tube were examined in light of their ability to repress the effects of co-injected Gli1 in vivo and the fact that their expression domains partially overlap (Lee et al., 1997). Injection of Gli2 or Gli3 RNAs into the dorsal quadrant of early frog embryos resulted in embryos displaying axial defects as compared to uninjected sibling controls (Fig. 3D). Within the ventral neural tube, injection of Gli2 or Gli3, but not a Gli3 zinc finger deletion mutant, resulted in the loss of HNF-3β from ventral neural tube cells (Fig. 3F,N; Table 1D), normally overlying the notochord (Fig. 3E,M). The expression of Shh mRNA was also analyzed in Gli3-injected embryos as a second ventral marker. Within the neural tube of early tadpoles, Shh is normally expressed in the ventrolateral diencephalon, the intrathalamic region and the floor plate (Fig. 3G,I; Ruiz i Altaba et al., 1995; Ekker et al., 1995). As with HNF-3β, embryos injected with Gli1 showed ectopic Shh expression (Lee et al., 1997) and those injected with Gli3 showed loss of its ventral expression in the floor plate and diencephalon (Fig. 3H,J), suggesting that Gli3 inhibits the differentiation of several, if not all, ventral cell types. The stronger effects in anterior regions reflects the prevalent localization of the injected proteins to this region after injection into the animal hemisphere and to the late development of posterior structures. All embryos scored displayed normal notochord differentiation as assessed morphologically or by gene/antigen expression. Cellular differentiation that is independent of Shh was not impeded in injected embryos. HNF-3β+/Shh+ neurons in the intrathalamic region/midbrain were observed in Gli3-injected embryos (Fig. 3H and not shown), consistent with their presence in u.v.-treated notochordless frog embryos (not shown). Moreover, Pax3 (Kim et al., 1997) was expressed at similar levels in the dorsal neural tube in control and injected embryos (Fig. 3K,L; n=8). Indeed, embryos ectopically expressing Gli3 in the dorsal neural tube after animal pole plasmid injections show normal morphological development (Lee et al., 1997). As with plasmid injections (Table 1C), coinjection of Gli1 plus Gli2 or Gli1 plus Gli3 RNAs, but not Gli1 plus a Gli3 zinc finger deletion mutant, resulted in a dramatic decrease in the incidence of ectopic HNF-3β expression (Table 1E). In addition, coinjected embryos also showed a marked decrease in the incidence of loss of endogenous HNF-3β. Thus, whereas Gli2 and Gli3 can antagonize the effects of Gli1 on ectopic floor plate induction, Gli1 can rescue their repression of endogenous floor plate differentiation. The differentiation of Nkx2.1/TTF-1+ ventral forebrain neurons is induced by Gli1 and repressed by Gli2 and Gli3 Floor plate cells do not differentiate in the forebrain. Shh and Gli1, however, are expressed in anterior ventral regions of the neural tube raising the possibility that, in these regions, Gli1 may induce the differentiation of distinct classes of Shhresponsive ventral neurons. To test this possibility, the expression of the Nkx2.1/TTF-1 homeoprotein (Lazzaro et al., 1991) was analyzed as a marker of ventral telencephalic and diencephalic secondary neurons (Fig. 4A,B). Nkx2.1/TTF-1 is induced by Shh in explant culture (Ericson et al., 1995) and is ectopically expressed in the dorsal forebrain in Gli1-injected embryos (Fig. 4C; Lee et al., 1997). Injection of either Gli3 or Gli2 failed to induce ectopic Nkx2.1/TTF-1+ neurons. Moreover, both were able to repress their normal differentiation (Fig. 4D,E and not shown). Gli3-injected embryos also showed the loss of the ventrolateral stripe of Shh expression in the diencephalon (Fig. 3H). Together, these results suggest that, in the forebrain, as in more posterior regions, Gli1 induces ventral cell differentiation whereas Gli2 and Gli3 not only lack this ability, but antagonize Gli1 function and the differentiation of Gli1-inducible ventral cell types. Motor neuron induction by Gli1 and Gli2 The repressive effect of Gli2 on Nkx2.1/TTF-1+ neurons contrasts with its involvement in endogenous neurogenesis (Brewster et al., 1998). In frog embryos, there are few specific markers described for neurons located at distinct D-V positions Pattern formation by combinatorial Gli gene function 2207 of the neural tube. The homeobox gene HB9, however, provides a clean marker of secondary spinal motor neurons in the tailbud and tadpole neural tube (Fig. 5A-C; Saha et al., 1997). Because Gli2 is expressed in the ventral region where motor neurons originate (Lee et al., 1997), the idea that Gli2 induces motor neuron development was tested. Gli2-injected tadpoles (stage ~32) displayed the ectopic expression of HB9 whereas control embryos showed only normal expression (Fig. 5F,G). Ectopic motor neurons were observed in the dorsal hindbrain and spinal cord but not in the forebrain or midbrain. Gli1 also induced ectopic HB9 expression (Fig. 5D,E). This result, however, is expected as injected Gli1 induces Gli2 expression. Embryos injected with frog (n=14) or human (n=23) Gli3 did not display ectopic HB9 expression (not shown). Ectopic HB9 expression was sometimes observed in epidermal ectoderm adjacent to the neural tube (not shown), indicating that Gli1/2 function can induce neuronal differentiation in non-neural ectoderm. This is consistent with previous findings demonstrating the neurogenic activity of Gli proteins (Brewster et al., 1998). The ability of Gli2 to induce motor neuron, but not floor plate development, together with its induction by Shh signaling, suggests that Gli2 mediates the Shh induction of motor neurons and possibly other ventral neuronal types. Gli3 represses motor neuron induction by Gli1 and Gli2 Gli2 is expressed throughout the neural plate, with the exception of the midline, and in dorsal as well as ventral regions in the ventricular zone of the neural tube (Lee et al., 1997; Figs 1C, 2D). Motor neurons, however, do not develop dorsally. If the Gli readout of a cell is important for determining cell type (Ruiz i Altaba, 1997), Gli3 acting as a repressor dorsally is predicted to account for the ventral restriction of the motor-neuron-inducing activity of Gli2. To test this possibility, synthetic Gli RNAs were co-injected and the embryos assayed for HB9 expression at the tadpole stage. Injection of frog Gli1 resulted in the induction of motor neurons (80% ectopic, n=5, Fig. 5) as did injection of frog Gli2 (79% ectopic, n=14, Fig. 5). In contrast, coinjection of frog Gli1 and frog or human Gli3 resulted in a marked inhibition of ectopic HB9 expression (50% ectopic, n=4 and 28% ectopic, n=18, respectively). Similarly, frog and human Gli3 were able to inhibit motor neuron induction by Gli2 (12% ectopic, n=17 and 4% ectopic, n=24, respectively). As with floor plate induction by Gli1, human Gli3 was more potent than frog Gli3 as a repressor of motor neuron differentiation. Ectopic expression of Gli3 in the ventral neural tube inhibited endogenous HB9 expression (n=5; not shown), although it is not clear whether this is a direct effect or mediated through the inhibition of endogenous floor plate differentiation (see Fig. 3N). Together, these results indicate that Gli3 inhibits floor plate and ventral forebrain neuron induction by Gli1 as well as motor neuron induction by Gli1 and Gli2, consistent with the role of Gli3 as a general repressor of Shh induction. DISCUSSION Gli proteins induce different programs of floor plate and neuronal development An unresolved question in vertebrate neural development is how progenitor cells choose a fate induced by Shh. Gli proteins are good candidates to participate in this process. In the frog neural plate, Gli1 is expressed in midline and immediately adjacent cells, responds to Shh signaling and induces floor plate cells and ventral neuronal types (Lee et al., 1997). An involvement of Gli1 in the differentiation of floor plate cells is suggested by three lines of evidence. First, all Gli proteins appear to recognize the same target sites. Second, co-injected Gli2 or Gli3 suppress ectopic floor plate induction by Gli1. Third, ectopic ventral expression of Gli2 or Gli3 suppresses endogenous floor plate and ventral forebrain neuronal differentiation. It remains possible, however, that injected Gli2 and Gli3 have additional effects that are distinct from the repression of Gli1 function. Gli1 may have a more general role in gliogenesis than just induction of floor plate cells as in the mouse spinal cord Gli1 is expressed in the ventral ventricular zone (Lee et al., 1997; Sasaki et al., 1997; Fig. 2) where oligodendrocyte precursors are found (Noll and Miller, 1993; Yu et al., 1994). A role of Gli1 in gliogenesis would be consistent with its initial isolation from a glioma line (Kinzler et al., 1987). Gli2, like Gli1, is induced by Shh but it is not expressed in midline cells. Gli2 may respond to low doses of Shh, with high levels at the midline repressing it. Alternatively, other midline factors may repress Gli2 expression. Notwithstanding how Gli2 is regulated, it is unable to induce floor plate development but it may mediate instead the induction of neuronal types located at a distance from the midline. This is supported by its neurogenic function (Brewster et al., 1998), the ectopic differentiation of HB9+ motor neurons in Gli2-injected embryos and the ability of co-injected Gli3 to inhibit ectopic motor neuron induction by Gli2. Gli1 also induces motor neurons although it may be indirect through its intermediate induction of floor plate cells and thus of Gli2. In the dorsal neural tube, the expression and function of Gli2 is likely to be independent of Shh suggesting that Gli2 responds to multiple cues. In contrast to Gli1 and Gli2, Gli3 is repressed by Shh and may be involved in the differentiation of Shh-independent dorsal neuronal types. A role for Gli3 in the dorsal neural tube is supported by its neurogenic activity (Brewster et al., 1998) and its requirement for normal development in mice and humans (Vortkamp et al., 1991; Hui and Joyner, 1993; Franz et al., 1994; Kang et al., 1997). Combined and antagonizing Gli gene function Gli proteins may balance each other’s functions in partially overlapping domains to create pattern. In this case, the ‘Gli readout’ of a cell is predicted to be critical for determining its fate (Ruiz i Altaba, 1997). Shh/Gli1 repress Gli3. At early gastrula stages prior to the onset of Shh expression in notochord precursors, low levels of Gli1 and Gli3 are detected in the dorsal animal cap, the prospective neural plate before midline differentiation (Fig. 6A; Lee et al., 1997). As the notochord begins to express Shh and the midline begins to express floor plate markers, Gli3 expression is absent from the midline, consistent with its repression by Shh from the forming notochord (Fig. 6B,C). A first step in ventralizing the medial neural plate by Shh may therefore be the repression of Gli3. Studies on limb development further suggest that Shh/Gli1 and Gli3 have 2208 A. Ruiz i Altaba mutually repressive relationships. Ectopic Shh expression in the anterior chick limb bud induces Gli1 and represses Gli3 (Marigo et al., 1996), whereas in Gli3 mutant mouse embryos, this region displays ectopic Shh expression (Masuya et al., 1995; Büscher et al., 1997). Similarly, Gli3 represses Shh/Gli1 in the dorsal neural tube. This implicates Gli3 and Shh/Gli1 in a mutually repressive interaction that appears to be critical for pattern formation in different tissues. Gli3 must be absent for ventral cell type induction and patterning and Gli3 is involved in repressing Shh in dorsal regions, thus allowing dorsal development. Gli2 and Gli3 have redundant functions in repressing floor plate induction by Gli1. However, Gli2 and Gli3 are differently regulated and have different functions in neuronal patterning as only Gli2 can induce spinal motor neurons. A partial redundancy between Gli2 and Gli3 has also been found in mouse skeletal patterning (Mo et al., 1997). Because the mutation introduced in the mouse Gli2 gene leaves intact the N-terminal region and the first two zinc fingers, it remains possible that such a mutation is not a null but rather a hypomorph if the N-terminal part of Gli proteins were to encode a repressive function like their Drosophila counterpart (Aza-Blanc et al., 1997). A regulatory feedback loop triggered by Shh may determine the identity and extent of the floor plate Gli2 represses the floor-plateinducing function of Gli1 and endogenous floor plate differentiation. Gli2 could therefore normally restrict the ability of Gli1 to induce floor plate development in cells immediately adjacent to the midline. This interaction could provide a molecular basis for the spatial restrictions to the propagation of floor plate induction previously observed (Ruiz i Altaba et al., 1995) that occurs before neural cells lose their competence to respond to floorplate-inducing signals (Placzek et al., 1993). Shh signaling from the Fig. 3. Gli1 induces and Gli2 and Gli3 repress floor plate development. (A) Normal expression of HNF-3β in the floor plate (fp), the midbrain (m) and zona limitans intrathalamica (zli) in a tadpole stage (stage ~34) embryo. The telencephalon (t) lacks expression. nt, notochord. (B,C) Ectopic expression of HNF-3β in tadpoles driven by injected hGli1 plasmids (B) resulting in a mosaic pattern, or synthetic fGli1 RNA (C), resulting in a more generalized expression. Arrows point to sites of ectopic expression. (D) Overall morphology of tadpoles (stage~34) injected with hGli3 RNA (bottom row) as compared with a normal sibling (top). Similar phenotypes were observed in fGli3 and fGli2 RNA-injected embryos (not shown). Injected embryos show deficiencies in the axis, head and face. (E,F) Normal expression of HNF-3β in the floor plate (fp) (E) and its loss in the anterior area of a sibling embryo injected with Gli3 (F, arrow). nc, notochord; nt, neural tube. (G,I) Expression of Shh in tadpole stage (stage ~34) embryos (n=32). (G) Shh is detected in the floor plate (fp), underlying notochord (nc), midbrain and zona limitans intrathalamica (zli/mb) and ventrolateral diencephalon (vdi). Shh is also expressed in the frontonasal process (fnp), pharyngeal endoderm/prechordal plate area (pp), branchial arches (ba) and hypochord. The cement gland is unlabeled and its position ventral to the frontonasal process is indicated. (I) high magnification of the trunk with expression of Shh in floor plate cells. (H,J) Expression of Shh in Gli3-injected tadpoles (stage ~34). (H) Expression of Shh in the floor plate and ventrolateral diencephalon is missing (arrows; 53%; n=17) whereas other sites appear normal taking into account the distortion of these embryos. (J) High magnification of the hindbrain region in which anterior floor plate cells are missing (arrow) but posterior Shh+ floor plate cells are present. Cells in the zli/m also express Shh (see above). The level of expression of Shh in the notochord in normal and injected embryos is similar. (K,L) Expression of Pax3 in the dorsal neural tube of both control (K; n=25) and Gli3 RNA-injected (L; 100%; n=14) tadpoles (stage~34). Pax3 is not expressed in the dorsal telencephalon. Both control and injected embryos also showed expression in the trigeminal ganglion and somites (not shown). cg, cement gland; dnt, dorsal neural tube; fb, forebrain. (M,N) Cross sections through the hindbrain of a normal embryo (M) and a sibling Gli3 RNA-injected embryo (N). HNF-3β expression within the neural tube (nt) in the nuclei of floor plate (fp) cells overlying the notochord (nc) is absent in Gli3-injected embryos which also display a thin ventral neural tube indicative of loss of ventral cell differentiation. Pattern formation by combinatorial Gli gene function 2209 notochord may thus initiate a regulatory cascade by inducing Gli1 and Gli2 expression in different yet overlapping domains. The identity of midline cells as floor plate may be determined by Gli1 in the absence of Gli2. In contrast, the mediolateral (dorsoventral) extent of the floor plate may be determined by Gli2 acting to antagonize the floor-plate-inducing function of Gli1. This interaction would occur in Gli1+/Gli2+ cells adjacent to the Gli1+ /Gli2− floor plate (Fig. 6B,C). The idea that Shh initiates a feedback regulatory loop that is critical for the patterning of induced cell types has a parallel with another signaling system. Recent experiments show that a class of inhibitory Smad proteins (Dad, Smad6, Smad7) prevents TGF-β superfamily signaling by competing with transducing Smads (e.g. Mad, Smad1, Smad2) for receptor binding (Tsuneizumi et al., 1997, Imamura et al., 1997, Nakao et al., 1997; Hayashi et al., 1997) or association with the general partner Smad4 (Hatta et al., 1998). Interestingly, the expression of Dad in Drosophila (Tsuneizumi et al., 1997) and Smad7 in mammalian cells (Nakao et al., 1997) is induced by Dpp and TGF-β signaling, respectively. Thus, inhibition of signaling by induced repressors that are members of the same family as the signal transducing molecules may be a general mechanism that provides temporal and/or spatial patterning information. Combinatorial function of Gli genes in neuronal patterning Different Gli proteins induce different types of neurons. Whereas Gli1 can induce a variety of ventral neuronal types including ventral forebrain neurons, hindbrain serotonergic neurons (Lee et al., 1997) and spinal motor neurons, Gli2 can only induce a subset of these ventral neuronal classes. However, in the posterior CNS, Gli1 may induce many ventral neuronal types through the intermediate induction of floor plate cells, which themselves will express Shh, thus inducing Gli2 in adjacent cells. Because Gli1 is restricted to midline and adjacent ventral cells, Gli2 is predicted to be normally involved in inducing secondary motor neuron differentiation in response to Shh. How then can Gli2 induce motor neurons only in the ventral region? Two strategies appear to play a role in restricting the motor-neuron-inducing Fig. 5. Motor neuron induction by Gli1 and Gli2. Expression of the homeobox gene HB9 in control (A-C) or injected (D-G) embryos at the tadpole stage (stage ~30-34). (A-C) Normal expression of HB9 in bilateral pools of motor neurons in the spinal cord (n=34) viewed dorsally (A), laterally (B) and in cross section through the cervical region (C). (D,F) Unilateral ectopic expression of HB9 in Gli1-injected embryos (91%, n=22) viewed in whole mount dorsally (D) or in cross section through the cervical spinal cord (F). (E,G) Ectopic expression of HB9 in Gli2-injected embryos (76%, n= 17) viewed laterally (E) and in cross section through the cervical region (G). Note that embryos were injected into one cell at the 2-cell stage and thus only one half of the neural tube is affected (denoted by a dashed line in F,G). In A,B,D,E, anterior is to the left. In C,F,G, dorsal is on top. de, dorsal endoderm; fp, floor plate; hb, hindbrain; mn, motor neurons; n, notochord; p, pituitary; sc, spinal cord. Arrows depict sites of ectopic expression. Fig. 4. Differential effects of Gli proteins on Nkx2.1/TTF-1+ ventral forebrain neurons. (A) Localization of Nkx2.1/TTF-1+ neurons in the ventral diencephalon (vdi) and ventral telencephalon (vt) of a tadpole (stage ~34; n=22). The antibody also recognizes a perinotochord (nc) antigen. (B,C) Normal (B) and ectopic (C) expression of Nkx2.1/TTF-1 in the forebrain of control and Gli1-injected embryos, respectively. The panels show forward views from the midbrain into the forebrain. Note the normal expression of Nkx2.1/TTF-1 in the ventral diencephalon (B) and its unilateral expansion to the dorsal forebrain (arrows in C) after injection of fGli1 RNA (78%, n=13). The dashed line depicts the axis of bilateral symmetry. (D,E) Loss of Nkx2.1/TTF-1 expression in the forebrain of Gli3-injected embryos. Arrows point to the anteroventral forebrain (fb). Note the normal differentiation of the notochord (nc) and the presence of a neural tube (nt) devoid of Nkx2.1/TTF-1 reactivity. Loss of Nkx2.1/TTF-1 expression was observed in fGli2 RNA-injected embryos (31% n=13; not shown), fGli3 RNA-injected embryos (43% n=14) and hGli3 RNA-injected embryos (not shown, 78% n=13). The posterior notochord is out of focus in these micrographs. function of Gli2 to the ventral neural tube. Ventrally, even though Gli2 is activated by Shh, midline factors repress its expression in prospective floor plate cells. Dorsally, Gli2 and Gli3 expression overlaps and, at least in equivalent amounts, Gli3 represses the motor-neuron-inducing function of Gli2. Thus, Gli2 is transcriptionally repressed in midline cells and 2210 A. Ruiz i Altaba A Gli1 Gli3 Animal cap B Neural plate Neural tube Gli3 Gli2 Gli1 M L Gli1 Gli2 Gli3 V D C Notochord Shh ? Gli1 Gli3 Neural plate Shh Fig. 6. Schematic diagram of Gli gene function and its regulation by Shh. (A) Diagram showing that, in the blastula and early gastrula animal cap, Gli3, but not Gli1, is expressed (Lee et al., 1997). Here, Gli3 is proposed to repress Gli1 and Shh. (B) Diagram representing the expression domains of the Gli genes in the neural plate (top) and in the ventricular zone of the neural tube (bottom). Only one half of the neural plate or neural tube is shown and the neural tube is represented as opened dorsally and flattened out to have a direct comparison with the neural plate. The x axis denotes mediolateral (M-L) position in the neural plate and dorsoventral (D-V) position in the neural tube. The y axis represents expression levels of the Gli genes. (C) During gastrulation and as the notochord underlies the midline of the neural plate, Shh secreted initially from the notochord induces expression of Gli1 in medial cells and that of Gli2 in more lateral cells. At the same time, it represses Gli3 transcription in a graded manner. Gli1 in medial cells induces floor plate (FP) development. Adjacent to the floor plate, Gli1 expression overlaps that of Gli2 where very low levels of Gli3 may also be present. These cells do not become floor plate but instead appear to be differentiating neurons (Lee et al., 1997). Gli2, and Gli3, may inhibit Gli1 from inducing floor plate differentiation in cells adjacent to the midline. Here Gli2 may induce the differentiation of ventral neurons (VN). The neurogenic abilities of Gli2 and Gli3 are also depicted by arrows and the inhibition of the motor-neuron-inducing ability of Gli2 by Gli3 is shown with a T bar. In dorsal regions combined Gli2/Gli3 function may induce dorsal neurons (DN). Factors inducing the dorsal expression of Gli3 and Gli2 are not known (?). Arrows represent positive actions and T bars repressive actions. Gli2 FP VN FP VN DN Neural plate Notochord functionally repressed in dorsal cells, leaving only ventral non-midline cells free of Gli2 inhibitors. Gli3 cannot induce ventral neuronal types and is predicted to induce intermediate and/or dorsal neurons. It is likely that Gli proteins may interact and synergize with other factors to create the fine pattern of neuronal types that arise in any one region of the neural tube. It remains possible that the graded distribution of Gli3 overlapping that of Gli2 may result in distinct Gli combinations that induce different neuronal fates. Testing this idea, however, awaits the availability of unambiguous markers of different cell types within the frog neural tube as well as determining whether Gli proteins, like Ci (Aza-Blanc et al., 1997), can yield varying forms with different activities. Posttranslational modification of Gli2 by Shh is hinted by the finding that Shh signaling is required in dividing motor neuron precursors up until late in the S phase of the last cell cycle before post-mitotic neurons are generated (Ericson et al., 1996). Thus, earlier transcriptional activation of Gli2 by Shh may be a requirement for motor neuron differentiation but it may not be sufficient. It is possible that the existence of motor-neuron-inducing Gli2 forms is dependent on sustained Shh signaling. In the forebrain, Gli2 and Gli3 also inhibit Gli1 function and Gli2 may normally induce the differentiation of a subset of ventral neurons. Gli2, however, is unable to induce ectopic differentiation of Nkx2.1/TTF-1+ neurons suggesting that, in this case, Gli1 acts directly. Independently of which classes of forebrain neurons Gli2 may induce, its repressive action on Nkx2.1/TTF-1+ cells raises the possibility that in the forebrain, like in more posterior CNS regions, a regulatory feedback loop triggered by Shh patterns the ventral region. More dorsally, the same kinds of interactions between Gli2 and Gli3 proposed for the hindbrain and spinal cord may also be involved in neuronal patterning. Gli gene function in the neural plate, and in the ventricular zone of the neural tube, may thus resemble the function of gap genes (Rivera-Pomar and Jäckle, 1996) during Drosophila anteroposterior patterning in that they may act to convert gradients into stripes. I thank Jeff Lee, Rachel Brewster, Nadia Dahmane, Gord Fishell, Ruth Lehmann, Ed Ziff and Will Talbot for discussion and/or comments on the manuscript. I am grateful to N. Dahmane for the mouse in situs, E. Storm for E12.5 Extra-toes homozygote mutant embryo bodies, A. Joyner for XtJ breeding mice, R. Di Lauro for the anti-TTF-1/Nkx2.1 antibody, M. Saha for the xHB9 cDNA and J. Lee for initial technical assistance. This work was supported by a Skirball Institute start-up grant, a Basil O’Connor award from the March of Dimes, a Pew Fellowship in the Biomedical Sciences and a grant from the NIH (RO1-NS 37352.01). REFERENCES Aza-Blanc, P., Ramírez-Weber, F.-A., Laget, M.-P., Schwartz, C. and Kornberg, T. B. (1997). Proteolysis that is inhibited by Hedgehog targets Pattern formation by combinatorial Gli gene function 2211 Cubitus Interruptus protein to the nucleus and converts it to a represor. Cell 89, 1043-1053. Brewster, R., Lee, J. and Ruiz i Altaba, A. (1998). Gli/Zic factors pattern the neural plate by defining domains of cell differentiation. Nature, in press. Büscher, D., Bosse, B., Heymer, J. and Rüther, U. (1997). Evidence for genetic control of Sonic hedgehog by Gli3 in mouse limb development. Mech. Dev. 62, 175-183. Chiang, C., Litingtung, Y., Lee, E., Young, K. E., Corden, J. L., Westphal, H. and Beachy, P. A. (1996). Cyclopia and defective axial patterning in mice lackingSonic hedgehog gene function. Nature 383, 407-413. Dickinson, M. E., Selleck, M. A. J., McMahon, A. P. and Bronner-Fraser, M. (1995). Dorsalization of the neural tube by the non-neural ectoderm. Development 121, 2099-2106. Eaton, S. and Kornberg, T. B. (1990). Repression of ci-D in posterior compartments of Drosophila by engrailed. Genes Dev. 4, 1068-1077. Echelard, Y., Epstein, D. J., St-Jacques, B., Shen, L, Mohler, J., McMahon, J. A. and McMahon, A. P. (1993). Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75, 1417-1430. Ekker, S. C., McGrew, L. L., Lai, C.-J., Lee, J. J., von Kessler, D. P., Moon, R. T. and Beachy, P. A. (1995). Distinct expression and shared activities of members of the hdgehog gene family of Xenopus laevis. Development 121, 2337-2347. Ericson, J., Muhr, J., Placzek, M., Lints, T., Jessell, T. M. and Edlund, T. (1995). Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell 81, 747-756. Eriscon, J., Morton, S., Kawakami, A., Roelink, H. and Jessell, T. M. (1996). Two critical periods of Sonic hedgehog signaling required for the specification of motor neuron identity. Cell 87, 661-673. Franz, T. (1994). Extra-toes (Xt) homozygous mutant mice demonstrate a role of the Gli-3 gene in the development of the forebrain. Acta Anat. 150, 3844. Harland, R. M. (1991). In situ hybridization: an improved whole mount method for Xenopus embryos. Meth. Enzymol. 36, 675-685. Hata, A., Lagna, G., Massagué, J. and Hemmati-Brivanlou, A. (1998). Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 12, 186-197. Hayashi, H., Abdollah, S., Qiu, Y., Cai, J., Xu, Y.-Y., Grinell, B. W., Richardson, M. A., Topper, J.N., Gimbrone, M. A., Wrana, J. L. and Falb, D. (1997). The MAD-related protein Smad7 associates with the TGFβ receptor and functions as an antagonist of TGFβ signaling. Cell 89, 11651173. Hughes, D. C., Allem, J., Morley, G., Sutherland, K., Ahmed, W., Prosser, J., Lettice, L., Allan, G., Mattei, M.-G., Farrall, M. and Hill, R. E. (1997). Cloning and sequencing of the mouse Gli2 gene: localization to the Dominant hemimelia critical region. Genomics 39, 205-215. Hui, C.-C. and Joyner, A. (1993). A mouse model of Greig cephalopolysyndatyly syndrome: the extra-toes mutation contains an intragenic deletion of the Gli3 gene. Nature Genetics 3, 241-246. Hui, C.-C., Slusarski, D., Platt, K. A., Holmgren, R. and Joyner, A. L. (1994). Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev. Biol. 162, 402-413. Hynes, M., Porter, J. A., Chians, C., Chang, D., Tessier-Lavigne, M. and Beachy, P. A. (1995). Induction of midbrain dopaminergic neurons by sonic hedgehog. Neuron 15, 35-44. Hynes, M., Stone, D. M., Dowd, M., Pitts-Meek, S., Goddard, A., Gurney, A. and Rosenthal, A. (1997). Control of cell pattern in the neural tube by the zinc finger transcription factor and oncogene Gli1. Neuron 19, 15-26. Imamura, T., Takese, M., Nishihara, A., Oeda, E., Hanai, J., Kawabata, M. and Miyazono, K. (1997). Smad6 inhibits signalling by the TGFβ superfamily. Nature 389, 622-626. Kang, S., Graham, J. M., Haskins Olney, A. and Biesecker, L. G. (1997). Gli3 frameshift mutations cause autosomal dominant Pallister-Hall syndrome. Nature Genetics 15, 266-268. Kim, P., Helms, A., Johnson, J. and Zimmerman, K. (1997). XATH-1, a vertebrate homolog of Drosophila atonal, induces neuronal differentiation within ectodermal precursors. Dev. Biol. 187, 1-12. Kinzler, K. W., Bigner, S. H., Bigner, D. D., Trent, J. M., Law, M. L., O’Brien, S. J., Wong, A. J. and Vogelstein, B. (1987). Identification of an amplified, highly expressed gene in a human glioma. Science 236, 70-73. Kinzler, K. W. and Vogelstein, B. (1990). The GLI gene encodes a nuclear protein which binds specific sequences in the human genome. Mol. Cell Biol. 10, 634-642. Krauss, S., Concordet, J.-P. and Ingham, P. W. (1993). A functionally conserved homolog of the Drosophila segment polarity gene hedgehog is expressed in tissues with polarizing activity in zebrafish embryos. Cell 75, 1431-1444. Lazzaro, A., Price, M., De Felioce, M. and Di Lauro, R. (1991). The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the fetal brain. Development 113, 1093-1104. Lee, J., Platt, K. A., Censullo, P. and Ruiz i Altaba, A. (1997). Gli1 is a target of sonic hedgehog that induces ventral neural tube development. Development 124, 2537-2552. Liem, K. F. Jr., Tremml, G., Roelink, H. and Jessell, T. M. (1995). Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm.Cell 82, 969-979. Marigo, V., Johnson, R. L., Vortkamp, A. and Tabin, C. J. (1996). Sonic hedgehogdifferentially regulates expression of Gli and Gli3 during limb development. Dev. Biol. 180, 273-283. Marine, J.-C., Bellefroid, E. J., Pendeville, H., Martial, J. A. and Pieler, T. (1997). A role for Xenopus Gli-type zinc finger proteins in the early embryonic patterning of mesoderm and neuroectoderm. Mech Dev. 63, 211225. Martí, E., Bumcrot, D. A., Takada, R. and McMahon, A. P. (1995). Requirement of 19K form of Sonic hedgehog for induction of distinct ventral cell types in CNS explants. Nature 375, 322-325. Masuya, H., Sagai, T., Wakana, S., Moriwaki, K. and Shiroishi, T. A. (1995). Duplicated zone of polarizing activity in polydactylous mouse mutants. Genes Dev. 13, 1645-1653. Mo, R., Freer, A. M., Zinyk, D. L., Crackower, M. A., Michaud, J., Heng, H. H. Q., Chik, K. W., Shi, X. M., Tsui, L. C., Cheng, S. H., Joyner, A. L. and Hui, C. C. (1997). Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development 124, 113-123. Moury, J. and Jacobson, A. (1989). Neural fold formation in newly created boundaries between neural plate and epidermis in the axolotl. Dev. Biol. 133, 44-57. Nakao, A., Afrakhte, M., Morén, A., Nakayama, T., Christian, J.L., Heuchel, R., Itoh, S., Kawabata, M., Heldin, N.-E., Heldin, C.-H. and ten Dijke, P. (1997). Identification of Smad7, a TGFβ-inducible antagonist of TGFβ signaling. Nature 389, 631-635. Nieuwkoop, P. D. and Faber, J. (1967). Normal Table of Xenopus laevis (Daudin). Amsterdam: North Holland. Noll, E. and Miller, R. H. (1993). Oligodendrocyte precursors originate at the ventral ventricular zone dorsal to the ventral midline regions in the embryonic rat spinal cord. Development 118, 563-573. Orenic, T. V., Slusarski, D. C., Kroll, K. L. and Holmgren, R. A. (1990). Cloning and characterization of the segment polarity gene cubitus interruptus dominant of Drosophila. Genes Dev. 4, 1053-1067. Pavletich, N. P. and Pabo, C. O. (1993). Crystal structure of a five-finger GLI-DNA complex: new perspectives on zinc fingers. Science 261, 17011707. Placzek, M., Jessell, T.M. and Dodd, J. (1993). Induction of floor plate differentiation by contact-dependent homeogenetic signals. Development 117, 205-218. Platt K. A., Michaud J. and Joyner A. L. (1997). Expression of the mouse Gli and Ptc genes is adjacent to embryonic sources of hedgehog signals suggesting a conservation of pathways between flies and mice. Development 62, 121-135. Riddle, R., Johnson, R. L., Laufer, E. and Tabin, C. (1993). Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 751, 401-1418. Rivera-Pomar, R. and Jäckle, H. (1996). From gradients to stripes in Drosophila embryogenesis: filling in the gaps. Trends Genet. 12, 478-483. Roelink, H., Augsburger, A., Heemskerk, J., Korzh, V., Norlin, S., Ruiz i Altaba, A., Tanabe, Y., Placzek, M., Edlund, T., Jessell, T. M. and Dodd, J. (1994). Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell 76, 761-775. Roelink, H., Porter, J. A., Chiang, C., Tanabe, Y., Chang, D. T., Beachy, P. A. and Jessell, T. M. (1995). Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell 81, 445-455. Ruiz i Altaba, A. (1993). In Essential Developmental Biology - A Practical Approach (ed. C. Stern and P. W. H. Holland). Oxford: IRL Press. Ruiz i Altaba, A. (1997). Catching a Gli-mpse of Hedgehog. Cell 90, 193-196. 2212 A. Ruiz i Altaba Ruiz i Altaba, A., Jessell, T. M. and Roelink, H. (1995). Restrictions to floor plate induction by hedgehog and winged helix genes in the neural tube of frog embryos. Mol. Cell. Neurosci. 6, 106-121. Rupp, R. A. W., Snider, L. and Weintraub, H. (1994). Xenopus embryos regulate nuclear localization of XmyoD. Genes Dev. 8, 1311-1323. Ruppert, J. M., Kinzler, K. W., Wong, A. J., Bigner, S. H., Kao, F. T., Law, M. L., Seuanez, H. N., O’Brien, S. J. and Vogelstein, B. (1988). The GLIKruppel family of human genes. Mol. Cell. Biol. 8, 3104-3113. Ruppert, J. M., Vogelstein, B., Arheden, K. and Kinzler, K. W. (1990). GLI3 encodes a 190 kilodalton protein with multiple regions of GLI similarity. Mol. Cell Biol. 10, 5408-5415. Saha, M. S., Miles, R. R. and Grainger, R. M. (1997). Dorsal-ventral patterning during neural induction in Xenopus: Assessment of spinal cord regionalization with xHB9, a marker for the motor neuron reigion. Dev. Biol. 187, 209-223. Sasaki, H., Hui, C. C., Nakafuku, M. and Kondoh, H. (1997). A binding site for Gli proteins is essential for HNF-3β floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development 124, 1313-1322. Tsuneizumi, K., Nakayama, T., Kamoshida, Y., Kornberg, T.,B., Christian, J.,L. and Tabata, T. (1997). Daughters against dpp modulates dpp organizing activity in Drosophila wing development. Nature 389, 627-631. Turner, D. L. and Weintraub, H. (1994). Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 8, 1434-1447. Vortkamp, A., Gessler, M. and Grzeschik, K.-H. (1991). Gli3 zinc-finger gene interrupted by translocations in Greig syndrome families. Nature 352, 539-540. Vortkamp, A., Gessler, M. and Grzeschik, K.-H. (1995). Identification of optimized target sequences for the GLI3 zinc finger protein. DNA Cell Biol. 14, 629-634. Walterhouse, D., Ahmed, M., Slusarski, D., Kalamaras, J., Boucher, D., Holmgren, R. and Iannaccone, P. (1993). gli, a zinc finger transcription factor and oncogene, is expressed during normal mouse development. Dev. Dyn. 196, 91-102. Yu, W.-P., Collarini, E. J., Pringle, N. P. and Richardson, W. D. (1994). Embryonic expression of myelin genes: evidence for a focal source of oligodendrocyte precursors in the ventricular zone of the neural tube. Neuron 12, 1353-1362.