* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Evasion of innate immunity by parasitic protozoa
Lymphopoiesis wikipedia , lookup
DNA vaccination wikipedia , lookup
Immune system wikipedia , lookup
Complement system wikipedia , lookup
Adaptive immune system wikipedia , lookup
Molecular mimicry wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Plasmodium falciparum wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Schistosoma mansoni wikipedia , lookup
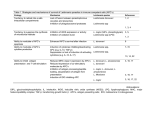
R EVIEW © 2002 Nature Publishing Group http://www.nature.com/natureimmunology Evasion of innate immunity by parasitic protozoa David Sacks and Alan Sher Parasitic protozoa are a major cause of global infectious disease. These eukaryotic pathogens have evolved with the vertebrate immune system and typically produce long-lasting chronic infections. A critical step in their host interaction is the evasion of innate immune defenses. The ability to avoid attack by humoral effector mechanisms, such as complement lysis, is of particular importance to extracellular parasites, whereas intracellular protozoa must resist killing by lysosomal enzymes and toxic metabolites.They do so by remodeling the phagosomal compartments in which they reside and by interfering with signaling pathways that lead to cellular activation. In addition, there is growing evidence that protozoan pathogens modify the antigen-presenting and immunoregulatory functions of dendritic cells, a process that facilitates their evasion of both innate and adaptive immunity. Parasitic protozoa are unicellular eukaryotic pathogens that dwell inside host cells and/or in extracellular fluids. These organisms typically produce long-lasting chronic infections in order to maximize their opportunities for successful transmission by contact with their intermediate hosts or release into the environment. Their success as parasites depends on a series of intricate and highly evolved host adaptations that enable them to evade destruction by the immune system. Protozoan infections are a major global health problem, affecting over half a billion people world wide. Several of the diseases they induce (such as malaria, African trypanosomiasis and visceral leishmaniasis) represent major causes of mortality and morbidity in tropical countries and, as such, are important impediments to economic development. No standardized vaccines exist for preventing any of the protozoan infections of humans, a situation attributed both to the complexity of the pathogens involved and their sophisticated strategies for evading host immune responses1. Protozoan parasites are highly adept at escaping the effects of adaptive humoral and cellular immunity. Perhaps the simplest solution for evading antibody responses is the adoption of an intracellular life style, as is done by Leishmania, Trypanosoma cruzi, Toxoplasma gondii and the liver and blood stages of malaria. Another major strategy—antigenic variation—protects extracellular protozoa such as African try- panosomes2 and Giardia3 and even malaria parasites4, which express their antigens on the surface of infected erythrocytes, from immune recognition. In addition, there is abundant evidence that protozoan infections actively regulate adaptive T cell responses, resulting in suppressed effector functions1. A striking example of this phenomenon is the recent demonstration that Leishmania major actively induces interleukin 10 (IL-10)–producing CD25+ T regulatory cells to prevent complete clearance of the parasite5. Although parasitic protozoa have provided some of the best studied paradigms of evasion of antibody- and T cell–mediated immunity by pathogens, a series of equally important adaptations occur during the initial establishment of infection, when parasitic invaders confront the innate immune system. The innate host defenses that the major blood and tissue protozoan pathogens of humans must penetrate, modify or avoid vary according to their mode of entry and the primary host tissue encountered (Table 1). These innate defenses include the epithelial barrier of the skin, the alternative complement cascade and other lytic serum components, lysosomal hydrolases and toxic oxygen metabolites of mononuclear phagocytes and the antigen-presentation and immunoregulatory functions of dendritic cells (DCs), which provide a crucial link with the adaptive immune response6. That protozoa have evolved specific mechanisms to evade these defenses is dramatically shown by the rapid destruction of preinfective vector-derived life cycle stages when artificially inoculated into mammalian hosts. These developmental adaptations, which enable blood and tissue protozoa to “run the gauntlet” of the vertebrate innate response, are the focus of our review. Evasion of humoral innate defenses Innate resistance to protozoa is mediated, in part, by pre-existing soluble factors that can potentially recognize and destroy invading parasites or target them for killing by effector cells. The alternative pathway of complement activation provides a first line of defense against extracellular parasites that must be subverted for infection to proceed. For example, whereas the epimastigote stage of T. cruzi found in insect vectors is susceptible to the alternative complement pathway, infective metacyclic and blood-stream trypomastigotes are resistant7. In this case, evasion of complement appears to be due to trypomastigote expression of a 160-kD glycoprotein (gp160), which is a homolog of the host complement-regulatory protein decay-accelerating factor (DAF)8. Like DAF, gp160 can bind to C3b and C4b and inhibit the uptake of subsequent members of the complement cascade, thus preventing convertase formation and lysis of the parasite. Importantly, whereas complement-sensitive epimastigotes fail to express gp160, epimastigotes transfected with gp160 are resistant to complement-mediated lysis9. Another interesting strategy is deployed by Leishmania species, for which the infective insect stage is transiently exposed to potentially Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892, USA. Correspondence should be addressed to D. S. ([email protected]). www.nature.com/natureimmunology • november 2002 • volume 3 no 11 • nature immunology 1041 R EVIEW © 2002 Nature Publishing Group http://www.nature.com/natureimmunology Table 1.The major protozoan pathogens of humans Parasite Vertebrate host habitat Mode of transmission Primary site of host invasion Malaria Leishmania Toxoplasma Intracellular (erythrocytes + hepatocytes) Intracellular (macrophages) Intracellular (macrophages, other nucleated cells) Intracellular (macrophages, muscle cells, other nucleated cells) and extracellular Extracellular Vector-born (mosquitoes) Vector-born (sand flies) Ingestion of parasite cysts Skin, blood (sporozoites) Skin Intestinal epithelial cells Vector-born (reduviid bugs) Skin, conjunctival mucosa Vector-born (tse-tse flies) Blood T. cruzi African trypanosomes lethal serum components after inoculation by pool-feeding phlebotomine vectors. Leishmania evade complement-mediated lysis while using complement activation as a mechanism for targeting host cells. When insect-stage procyclic promastigotes develop into infective metacyclic forms, their membrane is altered to prevent insertion of the lytic C5b-C9 membrane attack complex (MAC)10. This correlates with their expression of a modified surface lipophosphoglycan (LPG) that is approximately twice as long as the form on procyclic promastigotes, and which may act as a barrier for insertion of the MAC into the parasite surface membrane11. A further developmental change that occurs during generation of metacyclics is enhanced synthesis of the surface proteinase gp63, which can cleave C3b to the inactive iC3b form, thus preventing deposition of the lytic C5b-C9 complex12. However, iC3b also opsonizes the parasites for phagocytosis through the complement receptors CR3 and CR1, thereby targeting the parasite to the macrophage, its host cell of choice13. Conclusive evidence that LPG and gp63 are virulence factors has emerged from studies with L. major null-mutants that do not express these molecules; in each case these mutants were highly sensitive to complementmediated lysis and showed reduced virulence in BALB/c mice14,15. In addition to complement, protozoa must evade other soluble mediators of innate immunity in order to establish a foothold in the host. Perhaps the best studied are the primate-specific trypanosome lysis factors (TLFs)16, which contribute to the resistance of humans to infection with Trypanosoma brucei, an important pathogen of livestock. Biochemical analysis of the activity present in human serum revealed that high-density lipoproteins are part of the substance that mediates cytolysis of the parasite, and initial studies demonstrated that this complex, TLF1, is composed of several common apolipoproteins as well as a haptoglobin-related protein (Hpr)17,18. A second cytolytic complex, TLF2, has also been identified that shares many of the components of TLF1 but contains a distinct immunoglobulin M component and a lower lipid content19. Although the final lytic event has not been delineated, TLF must undergo receptor-mediated uptake and enter an intracellular acidic compartment for cytotoxicity to occur18. Whereas T. brucei is sensitive to TLF-mediated killing, the species that infect humans—T. brucei gambiense and T. brucei rhodesiense—are both refractory. This resistance is associated with a block in TLF endocytosis and retention of the complex in a structure known as the flagellar pocket16. The refractoriness of certain T. brucei rhodensiese strains to lysis also correlates with the expression of a serum resistance–associated (SRA) gene that is equivalent to truncated variant surface glycoprotein20. Importantly, transfection of SRA from T. brucei rhodesiense into T. brucei confers resistance to lysis by human serum; this argues that its expression may have been a critical step in the adaptation of an ancestral parasite for infection of primates21. Interestingly, antibodies raised against a homolog of SRA localize to the flagellar pocket, suggesting a possible role for the protein in TLF retention22. 1042 nature immunology • volume 3 no 11 • Remodeling host-cell compartments Parasitic protozoa that are adapted to an intracellular lifestyle must resist the antimicrobial mechanisms that can be induced in phagocytic and even nonphagoctyic host cells. Insofar as the acidified hydrolytic environment of host cell lysosomes represents the heart of the defensive machinery of many nucleated cells, the ability of acid-labile proteasesensitive parasites to avoid or modify this compartment is likely to be one key to their survival (Fig. 1). T. gondii resides in a phagosome that restricts its fusion with host endosomes and lysosomes. Toxoplasma actively penetrates both phagocytic and nonphagocytic cells, propelled by an actin-myosin–dependent gliding motility23. In the process, it establishes a nonfusigenic compartment, called the parasitophorous vacuole (PV), that lacks integral membrane proteins of host cell origin, but is extensively modified by secreted parasite proteins24,25. This remodeling seems to be crucial to the inhibition of PV acidification and lysosome fusion, as these events proceed normally after uptake of dead or opsonized parasites, which are internalized via classical receptormediated phagocytosis. T. cruzi trypomastigotes also actively invade mammalian cells but, unlike T. gondii, their cell entry is dependent not on the propulsive properties of the parasite, but on their ability to subvert a Ca2+-regulated lysosomal exocytic pathway23. The membrane of the parasitophorous vacuole is, therefore, derived from the membrane of lysosomes, and the vacuole itself remains acidic and potentially fusigenic with other lysosomes. T. cruzi growth and development cannot be sustained within this compartment; it depends instead on the ability of the parasite to escape rapidly into the cytosol. Exit from the vacuole is mediated by a parasite-secreted molecule, Tc-TOX, which has membrane pore-forming activity at acidic pH and is facilitated by a transsialidase present on the trypomastigote surface26,27. Interestingly, T. cruzi is unable to invade cells lacking transforming growth factor-β (TGF-β) receptors I or II28; this suggests that triggering this signaling pathway, perhaps by some parasite TNF-β homolog, might be needed to deactivate host cells during the early stages of infection. Leishmania parasites lack the machinery necessary for active invasion and are confined instead to professional phagocytes, mainly macrophages, with some exceptions (for example, fibroblasts, DCs and neutrophils)29. The uptake of Leishmania by macrophages proceeds via conventional receptor-mediated phagocytosis that involves a diversity of opsonic or pattern-recognition receptors (for example, CR3, CR1 and mannose fucose receptors)30 that are used depending on the species and stage of parasite and the presence or absence of fresh serum. Leishmania metacyclic promastigotes and amastigotes do not seem to remodel the phagosome in a major way because it rapidly fuses (within 30 min) with late endosomes or lysosomes31, generating a parasitophorous vacuole that maintains an acidic pH and hydrolytic activity. Using macrophage cell lines and unselected promastigotes, researchers have shown that LPG-repeating units can transiently inhibit phagosome maturation and that this delay may be necessary to allow november 2002 • www.nature.com/natureimmunology © 2002 Nature Publishing Group http://www.nature.com/natureimmunology R EVIEW sufficient time for promastigotes competitive inhibitor of the PKC Phagosome to differentiate into more hydroactivator DAG and/or by altering lase-resistant amastigotes32,33. the physical properties of the bilayer to inhibit PKC membrane These findings are consistent with T. cruzi trypomastigote translocation. These findings are the attenuation of L. major proagain consistent with impaired mastigote survival within macrointracellular survival of the L. phages after infection with targetmajor LPG–deficient mutant ed null mutants lacking LPG14. compared to the mutant in which The general significance of the Leishmania amastigote LPG expression was restored, transient inhibition of phagoalthough effects on specific sigsome-endosome fusion has been Parasitophorous naling events were not directly questioned, however, for two reavacuoles compared14. sons. First, Leishmania mexicana Leishmania promastigote mutants deficient in In addition to their innate promastigote Toxoplasma gondii LPG or other phosphoglycan-conmicrobicidal responses, macrotachyzoite Phagolysosome taining molecules survive equally phages can initiate the host actiLysosomes well as wild-type organisms withvation cascade by presenting antiin macrophages34, and second, gens and costimulatory molecules Figure 1. Remodeling of macrophage intracellular compartments by and by providing regulatory there is an absence of direct data parasitic protozoa. T. cruzi trypomastigotes enter the macrophage by induccytokines to T cells. A number of to indicate that mature promastiging the recruitment of lysosomes to the plasma membrane; they only transientstudies suggest mechanisms by otes are any less hydrolase-resisly reside in the parasitophorous vacuole before escape into the cytoplasm via which intracellular protozoa can tant than amastigotes. That secretion of a pore-forming molecule, termed Tc-TOX (yellow). T. gondii tachyzoites actively invade the cell and remodel a parasitophorous vacuole membrane interfere with the immune-initiaamastigotes are remarkably robust (blue) that contains secreted parasite proteins but excludes host proteins that tion functions of macrophages. is clear: the parasite does not would normally promote phagosome maturation, thereby preventing lysosome One of the more consistent and escape from the vacuole, but manfusion. Leishmania metacyclic promastigotes are taken up by receptor-mediated striking dysfunctions observed in ages to withstand the low pH and phagocytosis; phagosome maturation may be transiently inhibited by LPG macrophages infected with protoonslaught of acid hydrolases, pre(green), which becomes incorporated into the phagosome membrane.The replicating amastigote stage ultimately resides within a phagolysosome where they zoan parasites is their inability to sumably by producing an abunsurvive via production of cell-surface and secreted glycoconjugates, including produce IL-12, which—as the dance of cell surface and secreted GIPLS and proteophosphoglycan (PPG) (green). main physiological inducer of glyconjugates that protect the cell interferon-γ (IFN-γ) and T helper from proteolytic damage. As the L. mexicana phosphoglycan–deficient mutants were able to survive type 1 (TH1) cell differentiation—is an essential cytokine for the develnormally, it is likely that this species displays different or redundant opment of acquired resistance to most intracellular pathogens. In addivirulence determinants. It is possible, for example, that the huge para- tion, excess IL-12 production can lead to severe tissue damage and even sitophorous vacuole that is formed after infection with parasites of the mortality, a consequence that may also be detrimental to the parasite in L. mexicana complex might effectively dilute the hydrolases to which blocking its transmission cycle. It is no wonder then that protozoa have the parasite is exposed within the vacuole and obviate the need for cer- evolved a series of intricate and often redundant mechanisms for regutain surface or secreted glycans. lating IL-12 production by antigen-presenting cells, especially macrophages, which are the major potential source of the cytokine stimulated during early infection. Inhibition of host cell signaling pathways Infectious stages of Leishmania do not merely avoid IL-12 inducMacrophages possess primary defense mechanisms—including activation of macrophage oxidative metabolism and synthesis and release of tion, they actively and selectively inhibit it, leaving other pro-inflamarachidonic acid metabolites—that are induced by the attachment and matory cytokine or chemokine response pathways relatively intact41. engulfment of microbial agents. The major source of reactive oxygen Whereas the glycophosphatidylinositol (GPI) anchors of many paraintermediates (ROIs) in macrophages is the NADPH oxidase, a multi- sites are potent macrophage activators, Leishmania LPG and glycomponent enzyme that catalyzes the transfer of electrons from coinositol phospholipid (GIPL) inhibit IL-12p40 transcription while NADPH to molecular oxygen, resulting in the production of superox- failing to suppress TNF gene expression42. Although the receptors and ide and hydrogen peroxide35. It is generally believed that activation of signaling pathways involved in this selective inhibition of IL-12 synprotein kinase C (PKC) and protein tyrosine kinases (PTKs) are the two thesis have yet to be identified, suppression of NF-κB activity does not critical events involved in regulating phagocyte functions in response to appear to be involved. Because many IL-12 agonists that are inhibited a variety of extracellular stimuli. In vitro studies involving human cells in Leishmania-infected macrophages—including lipopolysaccharide have shown that macrophage functions, including phagocytosis and (LPS), CD40 ligand (CD40L) and especially IFN-γ—signal primarily ROI generation, are severely impaired after uptake of an insoluble through protein-tyrosine kinases, the observation that Janus kinase–sigdegraded host hemoglobin, called hemozoin, generated during blood- nal transducers and activators of transcription (Jak-STAT) signaling stage malarial infection36 (Fig. 2). These dysfunctions were attributed pathways are also inhibited in Leishmania-infected cells seems espeto inhibition of PKC translocation and activation in the hemozoin- cially relevant to the defective IL-12 response43. Defective phosphoryloaded cells37. Early studies suggested that Leishmania parasites also lation of Jak2 is attributed to rapid activation of a cytoplasmic protein avoid triggering the oxidative burst by actively inhibiting PKC activa- tyrosine phosphatase (PTP), SHP-144 (Fig. 2). SHP-1 is necessary for tion in macrophages38,39. Inhibition of PKC-mediated protein phospho- L. major survival insofar as SHP-1–deficient motheaten mice do not rylation was observed with purified LPG40, which might act either as produce lesions and their macrophages fail to support infection in www.nature.com/natureimmunology • november 2002 • volume 3 no 11 • nature immunology 1043 R EVIEW LPS TLR β Cytokine © 2002 Nature Publishing Group http://www.nature.com/natureimmunology α Jak cytb558 Hemozoin PKC RhoGDI Rac GDP O2 p67phox SHP1 MyD88 STAT IRAK ? IRAK IKK1 IKK2 p47phox p40phox Jak YP Y Y P CIS– P γ Leishmania TRAF6 STAT Y P P Y STAT MAP 3K Figure 2. Inhibition of macrophage signaling pathways. Inhibition of pathways by malaria-generated hemozoin or infection with Leishmania or T. gondii. Hemozoin and Leishmania impair the oxidative burst associated with phagocytosis by inhibiting the PKC activation required for assembly of the NADPH oxidase complex in its active form. Leishmania parasites also inhibit IFN-γ– or CD40L-induced PTKdependent signaling involved in IL-12 production by activation of the cellular phosphatase SHP-1 that inhibits Jak2 and STAT1 phosphorylation (P). Toxoplasma inhibits LPS-induced cytokine responses by inhibiting nuclear translocation of NF-κB and possibly phosphorylated STAT1. Toxoplasma O2– Y P STAT STAT Degradation of IκB Nucleus P Y IκB NF-κB Cytoplasm Induction of inflammatory and immune-response genes vitro45. Because specific ligation of the macrophage receptors CR1 and CR3 also leads to selective inhibition of IL-12 production—which, at least in the case of CR3, is associated with impaired tyrosine phosphorylation of STAT146—it is likely that host cell PTPs are rapidly induced by CR3 ligation during attachment and uptake of serum-exposed C3opsonized parasites. Given that Jak-STAT signaling pathways are involved in a broad range of macrophage responses, including induction of most proinflammatory cytokines, it is difficult to understand how Leishmania infection maintains such a selective effect on IL-12. One possible explanation lies in two observations: first, STAT1 regulates IFN consensus-binding protein (ICSBP) induction and—of the cytokines assayed—only activation of the IL-12p40 promoter appears to require ICSBP, and second, ICSBP knock-out mice (on resistant background) are susceptible to L. major infection47. The involvement of redundant STAT1-independent induction pathways for tumor necrosis factor-α (TNF-α), IL-1β and inducible nitric oxide synthase (iNOS) has been described (see below) and suggests a basis for the maintenance of these responses in Leishmania-infected cells. A similar mechanism may account for the down-regulation of IL-12p40 gene expression in mouse macrophages after uptake of Plasmodium berghei–infected erythrocytes48, which appear to be selective for IL-12 also and do not appear to be due to impaired NF-κB activation. The NF-κB family of transcription factors is an evolutionarily conserved group of proteins that are important in the regulation of numerous genes involved in innate and adaptive immunity—including those encoding IL-12, IFN-γ, TNF-α, iNOS and adhesion molecules—as well as those involved in cell proliferation and survival. Studies in T. gondii49,50 have demonstrated the ability of intracellular protozoa to actively interfere with the NF-κB–activation pathway in macrophages. In T. gondii–infected cells, despite rapid IκB phosphorylation and degradation, NF-κB fails to translocate to the nucleus. In contrast to Leishmania, T. gondii–induced response defects are more generalized and include both IL-12 and TNFα. With the use of virulent strains of T. gondii, the inhibition of NF-κB translocation in macrophages and its effect on iNOS expression was shown to be dependent on HS70 expression by the parasite that might impede nuclear transport by competing for access to nuclear pore complexes51. Disrupted nuclear transport of phosphorylated STATα has also been reported for T. gondii–infected macrophages and is thought to inhibit Jak-STAT–dependent MHC class II expression51 (Fig. 2). 1044 nature immunology • volume 3 no 11 • In addition to being directly suppressed by the parasite within infected cells, macrophage IL-12 production and effector functions can also be held in check by the down-modulatory cytokines IL-10 and TGF-β, which can themselves be up-regulated by the parasites or their products. Striking evidence in support of the importance of IL-10–dependent down-regulation of IL-12 production for both host and parasite comes from studies in IL-10–deficient mice infected with either T. gondii or T. cruzi. These animals display dysregulated IL-12 production and die of cytokine-associated tissue damage while showing decreased parasite burdens52,53. Similarly, low amounts of active TGF-β production by splenic mononuclear cells are associated with a lethal outcome in rodent malaria, and anti–TGF-β treatment transformed a normally resolving infection into a lethal one due to overproduction of pathogenic pro-inflammatory cytokines54. With respect to Leishmania, there is as yet no evidence that deficient production of these anti-inflammatory mediators is responsible for severe clinical outcomes. In contrast, there is ample evidence to suggest that overproduction of these cytokines contribute to uncontrolled parasite growth and nonhealing infections. Both IL-10 and TGF-β are produced by murine macrophages after Leishmania infection in vitro, and both promote intramacrophage replication and are important factors for determining in vivo susceptibility55,56. Finally, parasitic protozoa have evolved strategies to down-modulate signaling pathways leading to host cell apoptosis, thereby prolonging the life of the host cell and their own intracellular survival57. Induced apoptotic pathways in macrophages infected with either Leishmania donovani promastigotes58 or T. gondii tachyzoites59 were strongly inhibited, perhaps by parasite induced up-regulation of Bcl-2 homologs. Interestingly, the pro-apoptotic effects that T. cruzi infection has on both CD4+ and CD8+ T cells indirectly enhanced parasite growth, as the binding of apoptotic lymphocytes to vitronectin receptors on macrophages triggered TGF-β production and a burst of parasite replication in infected cells60. Manipulation of DC function To the extent that pathogenic protozoa have learned to condition their initial encounter with the innate immune system as a means of manipulating the subsequent adaptive immune response, it has been important to extend these observations to effects on “professional” antigenpresenting cells. In contrast to primary targets of infection, such as november 2002 • www.nature.com/natureimmunology R EVIEW Infection of monocytederived human DCs with T. cruzi trypomastigotes also Parasite Inhibitory signal Immunologic effects inhibits LPS-induced secreLeishmania Live infection (L. amazonensis) Inhibition of DC IL-12 production with turn on of IL-476 tion of pro-inflammatory Parasite culture media or LPG Inhibition of DC or LC migration73,74 T. cruzi Live infection GIPL (or ceramide portion) Suppression of LPS-induced IL-12, TNF-α, HLA-DR and cytokines—including IL-12 CD40 expression in human DCs71,72 and TNF-α—as well as HLAT. gondii Parasite induction of host LXA4 in vivo Inhibition of CCR5-dependent IL-12 production by DCs80,81 DR and CD40 up-regulation71. P. falciparum Ligation of CD36 (or CD51) by parasitized Suppression of DC maturation and function67,68 Similar effects were observed erythrocytes when human DCs were exposed to T. cruzi–derived macrophages, DCs may be temporally removed from encounter with GIPL or to only the ceramide portion of the compound72, although parasites or their products and may respond distinctively. For example, nonphysiological high concentrations of both molecules were used in whereas IL-12 production is actively suppressed in macrophages this in vitro study. infected with L. major or T. gondii, DCs actively produce IL-12p40 in With respect to Leishmania, the spontaneous migration of murine response to the same parasites, both in vitro and in vivo61–65. It should be splenic DCs or Langerhans cells (LCs) was inhibited by L. major pronoted that in each case the parasite infection alone was not sufficient for mastigote–conditioned media or L. major LPGs, respectively; this sughigh IL-12p70 production in vitro and that endogenous agonists, such gested that their ability to transport antigen to or within lymphoid tisas CD40L or IFN-γ, were necessary as costimuli. The different out- sue might be impaired during infection73,74. These effects need to be comes of protozoan encounter on IL-12 production by macrophages interpreted, however, in the context of other studies (referred to above). versus DCs is consistent with the delayed, albeit effective, immune These studies involved infection of murine DCs with viable L. major response to these infections that is ultimately achieved. Apart from the promastigotes or amastigotes; the infected cells up-regulated MHC obvious parasite advantage that delayed onset of immunity brings to class II and costimulatory molecules, produced IL-12p40 and down bear, suppression of IL-12 production by infected macrophages may regulated E-cadherin, which indicated that they were competent for again represent a coadaptation that dampens their local activation while antigen transport and presentation61,75. Uptake of Leishmania amazoprotecting the host from cytokine shock. nensis amastigotes or metacyclic promastigotes by mouse bone marFor those parasitic infections associated with T cell control mecha- row–derived DCs (BMDCs) also induced up-regulated expression of nisms that are not simply delayed, but are persistently compromised, MHC class II, CD40, CD80 and CD86 but, in contrast to L. major, the impaired maturation and function of appropriate DC subsets might be infected DCs produced IL-4 and no IL-1276. This suggested that the more expected (Table 2). A particularly interesting example is the inhi- DCs were conditioned by the parasite to prime the pathogenic TH2 cells bition of DC maturation by the malaria parasite Plasmodium falci- that dominate the response in vivo. parum66. Malaria-infected erythrocytes bind to the surface of myeloid Studies involving yet other Leishmania species suggest a more pasDCs in vitro and markedly suppress the normal up-regulation of major sive strategy for immune evasion, in which their interactions with antihistocompatibility complex (MHC) class II molecules, adhesion mole- gen-presenting cells appear to proceed in a relatively silent fashion. cules (for example, ICAM-1) and costimulatory molecules (CD83 and Uptake of L. mexicana by mouse BMDCs was not sufficient to activate CD86) on the cells after stimulation with LPS. The resulting DCs were them in vitro, although their maturation response to other stimuli was severely impaired in their capacity to induce allogenic as well as anti- not impaired77. A similar species restriction in Leishmania-induced DC gen-specific primary and secondary T cell responses67. The receptors on activation was observed after uptake of metacyclic promastigotes by the surface of DCs mediating this inhibitory effect are CD36 and CD51, human myeloid DCs, which were efficiently primed for CD40Land their artificial ligation mimicked the suppression induced by infect- induced ILp70 secretion by L. major, but not by L. tropica or L. donoed erythrocytes68. Interestingly, the same receptors are also involved in vani78. These observations may be significant because whereas L. major the recognition of apoptotic cells and, when incubated with DCs, such produces self-limiting infections that are controlled in the skin, the cells trigger reduced secretion of IL-12 and increased production of IL- other species mentioned are capable of disseminating to the viscera or 10 by these antigen-presenting cells. The major parasite ligand for to other cutaneous sites. Given the well described polymorphisms in the CD36 binding in infected erythrocytes is thought to be a conserved surface and secreted glycan structures that are expressed by different domain of P. falciparum erythrocyte membrane protein 1 (PfEMP1), a Leishmania species79, it will be interesting to determine whether they molecule that undergoes antigenic variation. Thus, a nonadherent para- account—at least in part—for the differences in innate response patsite line, which does not express PfEMP1 antigens on the red cell sur- terns and the spectrum of clinical disease. face, failed to inhibit DC maturation. From the above discussion it would appear that parasites carefully The relevance of these observations to acute disease is indicated by regulate the induction of IL-12 from DCs during early infection as a the finding that the percentage of HLA-DR+ DCs was significantly means of determining the character of the adaptive immune response lower in children with severe or mild malaria compared to healthy con- that eventually decides their fate in the host. In addition, there is emergtrols68. At present there is no direct evidence that impaired DC matura- ing evidence for the existence of distinct parasite-triggered pathways tion in children with acute malaria is CD36-mediated or that this event for regulating DC IL-12 production once it has been initiated. One such is at all necessary for successful malaria infection. Nevertheless, it is mechanism has been identified by studies of IL-12 synthesis by murine intriguing that infected erythrocytes with high CD36 binding affinity splenic DCs after injection of a soluble extract of T. gondii tachyzoites are more frequently observed in isolates from patients with mild as (STAg). The response was short-lived and could not be recalled by a opposed to severe disease69,70. It suggests that adhesion of infected ery- second injection of STAg for a period of 1 week after initial priming80. throcytes to CD36 may suppress the pro-inflammatory response to the This “paralysis” of the DC IL-12 response induced by STAg does not parasite, an outcome that would favor both host and parasite. require IL-10 but instead appears to depend on the induction of lipoxin © 2002 Nature Publishing Group http://www.nature.com/natureimmunology Table 2. Impairment of DC function by parasitic protozoa www.nature.com/natureimmunology • november 2002 • volume 3 no 11 • nature immunology 1045 © 2002 Nature Publishing Group http://www.nature.com/natureimmunology R EVIEW A4 (LXA4), a product of arachadonic metabolism81. This eicosonoid appears to function by suppressing CCR5 expression, a chemokine receptor involved in IL-12 stimulation by STAg. The same mechanism of IL-12 suppression is likely to operate during natural infection because T. gondii–infected mice with a defect in an enzyme (5-LO) required for LXA4 show excess IL-12 production and, in common with infected IL-10–deficient mice53, succumb to cytokine shock and show decreased parasite burdens (J. Aliberti et al., unpublished data). Taken together, these findings suggest that once IL-12 production by DCs has been induced and TH1 responses initiated, synthesis of the cytokine is actively down-regulated, probably to the joint benefit of both host and parasite. Whether analogous mechanisms suppress the IL-12 responses of DCs to other protozoa remains to be determined. Concluding comments In learning to evade innate host defenses, protozoan parasites appear to have mastered the intricacies of both cell biology and cellular immunology. As such, they can teach us important lessons about both fields of biology as well as their points of intersection. In particular, the strategies used by these pathogens for remodeling cellular compartments offer insights into the dynamics of host membranes and intracellular trafficking. At the same time there is much to be learned about host-signaling pathways and immune-response initiation and polarization by studying the mechanisms used by protozoa to suppress or divert immune responses. Because innate defenses (for example, lysis by complement or TLF) have the potential to block infection, the mechanisms used to evade them are potential “Achilles’ heels”, and offer newly identified and largely unexploited targets for vaccine and/or pharmacologic intervention. 1. Pearce, E. J., Scott, P. A. & Sher, A. in Fundamental immunology (ed. Paul,W.) 1271–1294 (LippincottRaven, Philadelphia, 1999). 2. Borst, P. et al. Antigenic variation in trypanosomes. Arch. Med. Res. 27, 379–388 (1996). 3. Nash,T. E. Surface antigenic variation in Giardia lamblia. Mol. Microbiol. 45, 585–590 (2002). 4. Kyes, S., Horrocks, P. & Newbold, C. Antigenic variation at the infected red cell surface in malaria. Annu. Rev. Microbiol. 55, 673–707 (2001). 5. Belkaid,Y. et al. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 194, 1497–506 (2001). 6. Hunter, C. & Sher, A. in Immunology of Infectious Diseases (eds. Kaufman, S., Sher, A. & Ahmed, R.) 111–126 (ASM Press,Washington DC, 2001). 7. Joiner, K. A. Complement evasion by bacteria and parasites. Annu. Rev. Microbiol. 42, 201–230 (1988). 8. Norris, K. A., Bradt, B., Cooper, N. R. & So, M. Characterization of a Trypanosoma cruzi C3 binding protein with functional and genetic similarities to the human complement regulatory protein, decayaccelerating factor. J. Immunol. 147, 2240–2247 (1991). 9. Norris, K. A. Stable transfection of Trypanosoma cruzi epimastigotes with the trypomastigote-specific complement regulatory protein cDNA confers complement resistance. Infect. Immun. 66, 2460–2465 (1998). 10. Puentes, S. M., Da Silva, R. P., Sacks, D. L., Hammer, C. H. & Joiner, K. A. Serum resistance of metacyclic stage Leishmania major promastigotes is due to release of C5b-9. J. Immunol. 145, 4311–4316 (1990). 11. McConville, M. J.,Turco, S. J., Ferguson, M. A. & Sacks, D. L. Developmental modification of lipophosphoglycan during the differentiation of Leishmania major promastigotes to an infectious stage. EMBO J. 11, 3593–3600 (1992). 12. Brittingham, A. et al. Role of the Leishmania surface protease gp63 in complement fixation, cell adhesion, and resistance to complement-mediated lysis. J. Immunol. 155, 3102–3111 (1995). 13. Mosser, D. M. & Edelson, P. J.The mouse macrophage receptor for C3bi (CR3) is a major mechanism in the phagocytosis of Leishmania promastigotes. J. Immunol. 135, 2785–2789 (1985). 14. Spath, G. F. et al. Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc. Natl. Acad. Sci. USA 97, 9258–9263 (2000). 15. Joshi, P. B., Kelly, B. L., Kamhawi, S., Sacks, D. L. & McMaster,W. R.Targeted gene deletion in Leishmania major identifies leishmanolysin (GP63) as a virulence factor. Mol. Biochem. Parasitol. 120, 33–40 (2002). 16. Raper, J., Portela, M. P., Lugli, E., Frevert, U. & Tomlinson, S.Trypanosome lytic factors: novel mediators of human innate immunity. Curr. Opin. Microbiol. 4, 402–408 (2001). 17. Hajduk, S. L. et al. Lysis of Trypanosoma brucei by a toxic subspecies of human high density lipoprotein. J. Biol. Chem. 264, 5210–5217 (1989). 18. Smith, A. B., Esko, J. D. & Hajduk, S. L. Killing of trypanosomes by the human haptoglobin-related protein. Science 268, 284–286 (1995). 19. Raper, J., Fung, R., Ghiso, J., Nussenzweig,V. & Tomlinson, S. Characterization of a novel trypanosome lytic factor from human serum. Infect. Immun. 67, 1910–1916 (1999). 20. De Greef, C. & Hamers, R.The serum resistance-associated (SRA) gene of Trypanosoma brucei rhodesiense encodes a variant surface glycoprotein-like protein. Mol. Biochem. Parasitol. 68, 277–284 (1994). 21. Xong, H.V. et al. A VSG expression site-associated gene confers resistance to human serum in Trypanosoma rhodesiense. Cell 95, 839–846 (1998). 22. Milner, J. D. & Hajduk, S. L. Expression and localization of serum resistance associated protein in 1046 nature immunology • volume 3 no 11 • Trypanosoma brucei rhodesiense. Mol. Biochem. Parasitol. 104, 271–283 (1999). 23. Sibley, L. D. & Andrews, N.W. Cell invasion by un-palatable parasites. Traffic 1, 100–106 (2000). 24. Mordue, D. G., Desai, N., Dustin, M. & Sibley, L. D. Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. J. Exp. Med. 190, 1783–1792 (1999). 25. Lingelbach, K. & Joiner, K. A.The parasitophorous vacuole membrane surrounding Plasmodium and Toxoplasma: an unusual compartment in infected cells. J. Cell Sci. 111, 1467–1475 (1998). 26. Andrews, N.W., Abrams, C. K., Slatin, S. L. & Griffiths, G. A T. cruzi-secreted protein immunologically related to the complement component C9: evidence for membrane pore–forming activity at low pH. Cell 61, 1277–1287 (1990). 27. Hall, B. F.,Webster, P., Ma, A. K., Joiner, K. A. & Andrews, N.W. Desialylation of lysosomal membrane glycoproteins by Trypanosoma cruzi: a role for the surface neuraminidase in facilitating parasite entry into the host cell cytoplasm. J. Exp. Med. 176, 313–325 (1992). 28. Ming, M., Ewen, M. E. & Pereira, M. E.Trypanosome invasion of mammalian cells requires activation of the TGF-β signaling pathway. Cell 82, 287–296 (1995). 29. Rittig, M. G. & Bogdan, C. Leishmania-host-cell interaction: complexities and alternative views. Parasitol.Today 16, 292–297 (2000). 30. Alexander, J. & Russell, D. G.The interaction of Leishmania species with macrophages. Adv. Parasitol. 31, 175–254 (1992). 31. Courret, N. et al. Biogenesis of Leishmania-harbouring parasitophorous vacuoles following phagocytosis of the metacyclic promastigote or amastigote stages of the parasites. J. Cell Sci. 115, 2303–2316 (2002). 32. Desjardins, M. & Descoteaux, A. Inhibition of phagolysosomal biogenesis by the Leishmania lipophosphoglycan. J. Exp. Med. 185, 2061–2068 (1997). 33. Dermine, J. F., Scianimanico, S., Prive, C., Descoteaux, A. & Desjardins, M. Leishmania promastigotes require lipophosphoglycan to actively modulate the fusion properties of phagosomes at an early step of phagocytosis. Cell Microbiol. 2, 115–126 (2000). 34. Ilg,T., Demar, M. & Harbecke, D. Phosphoglycan repeat-deficient Leishmania mexicana parasites remain infectious to macrophages and mice. J. Biol. Chem. 276, 4988–4997 (2001). 35. Nathan, C. & Shiloh, M. U. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 97, 8841–8848 (2000). 36. Schwarzer, E. et al. Impairment of macrophage functions after ingestion of Plasmodium falciparuminfected erythrocytes or isolated malarial pigment. J. Exp. Med. 176, 1033–1041 (1992). 37. Schwarzer, E.,Turrini, F., Giribaldi, G., Cappadoro, M. & Arese, P. Phagocytosis of P. falciparum malarial pigment hemozoin by human monocytes inactivates monocyte protein kinase C. Biochim. Biophys. Acta 1181, 51–54 (1993). 38. Moore, K. J., Labrecque, S. & Matlashewski, G. Alteration of Leishmania donovani infection levels by selective impairment of macrophage signal transduction. J. Immunol. 150, 4457–4465 (1993). 39. Olivier, M., Brownsey, R.W. & Reiner, N. E. Defective stimulus-response coupling in human monocytes infected with Leishmania donovani is associated with altered activation and translocation of protein kinase C. Proc. Natl. Acad. Sci. USA 89, 7481–7485 (1992). 40. Descoteaux,A., Matlashewski, G. & Turco, S. J. Inhibition of macrophage protein kinase C-mediated protein phosphorylation by Leishmania donovani lipophosphoglycan. J. Immunol. 149, 3008–3015 (1992). 41. McDowell, M. A. & Sacks, D. L. Inhibition of host cell signal transduction by Leishmania: observations relevant to the selective impairment of IL-12 responses. Curr. Opin. Microbiol. 2, 438–443 (1999). 42. Piedrafita, D. et al. Regulation of macrophage IL-12 synthesis by Leishmania phosphoglycans. Eur. J. Immunol. 29, 235–244 (1999). 43. Nandan, D. & Reiner, N. E. Attenuation of γ interferon-induced tyrosine phosphorylation in mononuclear phagocytes infected with Leishmania donovani: selective inhibition of signaling through Janus kinases and Stat1. Infect. Immun. 63, 4495–4500 (1995). 44. Blanchette, J., Racette, N., Faure, R., Siminovitch, K. A. & Olivier, M. Leishmania-induced increases in activation of macrophage SHP-1 tyrosine phosphatase are associated with impaired IFN-γ-triggered Jak2 activation. Eur J. Immunol. 29, 3737–3744 (1999). 45. Forget, G. et al. Role of host phosphotyrosine phosphatase SHP-1 in the development of murine Leishmaniasis. Eur. J. Immunol. 31, 3185–3196 (2001). 46. Marth,T. & Kelsall, B. L. Regulation of interleukin-12 by complement receptor 3 signaling. J. Exp. Med. 185, 1987–1995 (1997). 47. Giese, N. A. et al. Interferon (IFN) consensus sequence-binding protein, a transcription factor of the IFN regulatory factor family, regulates immune responses in vivo through control of interleukin 12 expression. J. Exp. Med. 186, 1535–1546 (1997). 48. Xu, X. et al. Down-regulation of IL-12 p40 gene in Plasmodium berghei-infected mice. J. Immunol. 167, 235–241 (2001). 49. Butcher, B. A., Kim, L., Johnson, P. F. & Denkers, E.Y.Toxoplasma gondii tachyzoites inhibit proinflammatory cytokine induction in infected macrophages by preventing nuclear translocation of the transcription factor NF-κB. J. Immunol. 167, 2193–2201 (2001). 50. Shapira, S., Speirs, K., Gerstein, A., Caamano, J. & Hunter, C. A. Suppression of NF-κB activation by infection with Toxoplasma gondii. J. Infect. Dis. 185 (Suppl.) 66–72 (2002). 51. Dobbin, A., Smith, N. C. & Jonson, A. M. Heat shock protein 70 is a potential virulence factor in murine Toxoplasma infection via immunomodulation of host NF-κB and nitric oxide. J. Immunol. 169, 958–965 (2002). 52. Neyer, L. E. et al. Role of interleukin-10 in regulation of T-cell-dependent and T-cell- independent mechanisms of resistance to Toxoplasma gondii. Infect. Immun. 65, 1675–1682 (1997). 53. Gazzinelli, R.T. et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ and TNF-α. J. Immunol. 157, 798–805 (1996). 54. Omer, F. M., Kurtzhals, J. A. & Riley, E. M. Maintaining the immunological balance in parasitic infections: a role for TGF-β? Parasitol.Today 16, 18–23 (2000). 55. Kane, M. M. & Mosser, D. M.The role of IL-10 in promoting disease progression in Leishmaniasis. J. Immunol. 166, 1141–1147 (2001). 56. Barral, A. et al. Transforming growth factor β as a virulence mechanism for Leishmania braziliensis. Proc. Natl. Acad. Sci. USA 90, 3442–3446 (1993). 57. Luder, C. G., Gross, U. & Lopes, M. F. Intracellular protozoan parasites and apoptosis: diverse strategies to modulate parasite-host interactions. Trends Parasitol. 17, 480–486 (2001). 58. Moore, K. J.,Turco, S. J. & Matlashewski, G. Leishmania donovani infection enhances macrophage viability in the absence of exogenous growth factor. J. Leukoc. Biol. 55, 91–98 (1994). 59. Nash, P. B. et al. Toxoplasma gondii-infected cells are resistant to multiple inducers of apoptosis. J. Immunol. 160, 1824–1830 (1998). 60. Freire-de-Lima, C. G. et al. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature 403, 199–203 (2000). 61. von Stebut, E., Belkaid,Y., Jakob,T., Sacks, D. L. & Udey, M. C. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications november 2002 • www.nature.com/natureimmunology © 2002 Nature Publishing Group http://www.nature.com/natureimmunology R EVIEW for the initiation of anti-Leishmania immunity. J. Exp. Med. 188, 1547–1552 (1998). 62. Marovich, M. A., McDowell, M. A.,Thomas, E. K. & Nutman,T. B. IL-12p70 production by Leishmania major-harboring human dendritic cells is a CD40/CD40 ligand-dependent process. J. Immunol. 164, 5858–5865 (2000). 63. Gorak, P. M., Engwerda, C. R. & Kaye, P. M. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur. J. Immunol. 28, 687–695 (1998). 64. Subauste, C. S. & Wessendarp, M. Human dendritic cells discriminate between viable and killed Toxoplasma gondii tachyzoites: dendritic cell activation after infection with viable parasites results in CD28 and CD40 ligand signaling that controls IL-12-dependent and -independent T cell production of IFN-γ. J. Immunol. 165, 1498–505 (2000). 65. Scanga, C. A. et al. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 168, 5997–6001 (2002). 66. Urban, B. C. & Roberts, D. J. Malaria, monocytes, macrophages and myeloid dendritic cells: sticking of infected erythrocytes switches off host cells. Curr. Opin. Immunol. 14, 458–465 (2002). 67. Urban, B. C. et al. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 400, 73–77 (1999). 68. Urban, B. C.,Willcox, N. & Roberts, D. J. A role for CD36 in the regulation of dendritic cell function. Proc. Natl. Acad. Sci. USA 98, 8750–8755 (2001). 69. Newbold, C. et al. Receptor-specific adhesion and clinical disease in Plasmodium falciparum. Am. J.Trop. Med. Hyg. 57, 389–398 (1997). 70. Rogerson, S. J. et al. Cytoadherence characteristics of Plasmodium falciparum-infected erythrocytes from Malawian children with severe and uncomplicated malaria. Am. J.Trop. Med. Hyg. 61, 467–472 (1999). www.nature.com/natureimmunology • november 2002 71. Van Overtvelt, L. et al. Trypanosoma cruzi infects human dendritic cells and prevents their maturation: inhibition of cytokines, HLA-DR, and costimulatory molecules. Infect. Immun. 67, 4033–4040 (1999). 72. Brodskyn, C. et al. Glycoinositolphospholipids from Trypanosoma cruzi interfere with macrophages and dendritic cell responses. Infect. Immun. 70, 3736–3743 (2002). 73. Jebbari, H., Stagg, A. J., Davidson, R. N. & Knight, S. C. Leishmania major promastigotes inhibit dendritic cell motility in vitro. Infect. Immun. 70, 1023–1026 (2002). 74. Ponte-Sucre, A., Heise, D. & Moll, H. Leishmania major lipophosphoglycan modulates the phenotype and inhibits migration of murine Langerhans cells. Immunology 104, 462–467 (2001). 75. Konecny, P. et al. Murine dendritic cells internalize Leishmania major promastigotes, produce IL-12 p40 and stimulate primary T cell proliferation in vitro. Eur. J. Immunol. 29, 1803–1811 (1999). 76. Qi, H., Popov,V. & Soong, L. Leishmania amazonensis-dendritic cell interactions in vitro and the priming of parasite-specific CD4+ T cells in vivo. J. Immunol. 167, 4534–4542 (2001). 77. Bennett, C. L., Misslitz, A., Colledge, L., Aebischer,T. & Blackburn, C. C. Silent infection of bone marrow-derived dendritic cells by Leishmania mexicana amastigotes. Eur. J. Immunol. 31, 876–883 (2001). 78. McDowell, M. A., Marovich, M., Lira, R., Braun, M. & Sacks, D. Leishmania priming of human dendritic cells for CD40 ligand-induced interleukin-12p70 secretion is strain and species dependent. Infect. Immun. 70, 3994–4001 (2002). 79. Turco, S. J., Spath, G. F. & Beverley, S. M. Is lipophosphoglycan a virulence factor? A surprising diversity between Leishmania species. Trends Parasitol. 17, 223–226 (2001). 80. Reis e Sousa, C. et al. Paralysis of dendritic cell IL-12 production by microbial products prevents infection-induced immunopathology. Immunity 11, 637–647 (1999). 81. Aliberti, J., Hieny, S., Reis e Sousa, C., Serhan, C. N. & Sher,A. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nature Immunol. 3, 76–82 (2002). • volume 3 no 11 • nature immunology 1047